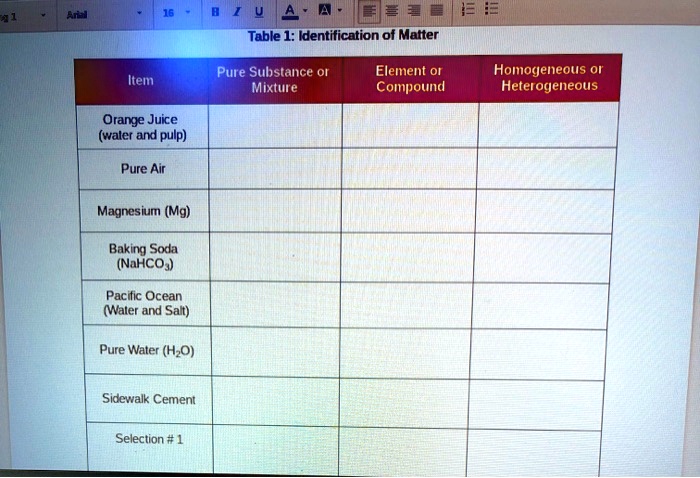
Orange juice with pulp pure substance or mixture
A Orange juice contains particles of solid pulp as well as liquid; it is not chemically pure. B Because its composition is not uniform throughout, orange juice is a heterogeneous mixture. What is this? In the case of orange juice, whether it is fresh graceeecharisss right from the fruit, or whether it is made from concentrate, the substance is a mixture.
Use app Login. Why is orange juice considered a solution? Doesn't it have pulp in ti so it's a suspension? Open in App. Verified by Toppr.
Orange juice with pulp pure substance or mixture
Chemists study the structures, physical properties, and chemical properties of material substances. These consist of matter , which is anything that occupies space and has mass. Gold and iridium are matter, as are peanuts, people, and postage stamps. Smoke, smog, and laughing gas are matter. Energy, light, and sound, however, are not matter; ideas and emotions are also not matter. The mass of an object is the quantity of matter it contains. Mass is a fundamental property of an object that does not depend on its location. In physical terms, the mass of an object is directly proportional to the force required to change its speed or direction. A more detailed discussion of the differences between weight and mass and the units used to measure them is included in Essential Skills 1 Section 1. Weight, on the other hand, depends on the location of an object. An astronaut whose mass is 95 kg weighs about lb on Earth but only about 35 lb on the moon because the gravitational force he or she experiences on the moon is approximately one-sixth the force experienced on Earth. For practical purposes, weight and mass are often used interchangeably in laboratories. Under normal conditions, there are three distinct states of matter: solids, liquids, and gases.
If all portions of a material are in the same state, have no visible boundaries, and are uniform throughout, then the material is homogeneous. Milk, for example, appears to be homogeneous, but when examined under a microscope, it clearly consists of tiny globules of fat and protein dispersed in water.
No, orange juice is not considered a pure substance. Orange juice is a heterogeneous mixture, but it could also be processed into a homogeneous mixture. But scientifically and chemically speaking, the purity of a substance at the grocer level is far different. If there are other building blocks that were not bonded to each other, the substance cannot be called a pure substance. When you squeeze an orange, the juice comes out. It seems like the liquid from the fruit is what it is, right? Orange juice is actually made up of multiple substances, including but not limited to: water, pectin, organic acids, sugars, and phenolic compounds.
No, orange juice is not considered a pure substance. Orange juice is a heterogeneous mixture, but it could also be processed into a homogeneous mixture. But scientifically and chemically speaking, the purity of a substance at the grocer level is far different. If there are other building blocks that were not bonded to each other, the substance cannot be called a pure substance. When you squeeze an orange, the juice comes out. It seems like the liquid from the fruit is what it is, right? Orange juice is actually made up of multiple substances, including but not limited to: water, pectin, organic acids, sugars, and phenolic compounds. Orange juice is made up of more building blocks than is worth delving into for the purpose of answering this question.
Orange juice with pulp pure substance or mixture
When we speak of a pure substance , we are speaking of something that contains only one kind of matter. This can either be one single element or one single compound, but every sample of this substance that you examine must contain exactly the same thing with a fixed, definite set of properties. Compounds are made up of one or more element. If we take two or more pure substances and mix them together, we refer to this as a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their individual properties. For example sodium is a soft shiny metal and chlorine is a pungent green gas. These two elements can combine to form the compound, sodium chloride table salt which is a white, crystalline solid having none of the properties of either sodium or chlorine. If, however, you mixed table salt with ground pepper, you would still be able to see the individual grains of each of them and, if you were patient, you could take tweezers and carefully separate them back into pure salt and pure pepper. A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture.
Capybara clicker unblocked
Gravity does not pull them down or out. Whereas the volume of gases strongly depends on their temperature and pressure the amount of force exerted on a given area , the volumes of liquids and solids are virtually independent of temperature and pressure. Mixtures of two or more liquids with different boiling points can be separated with a more complex distillation apparatus. But not without a lot of effort. Another example is the distillation of alcoholic spirits such as brandy or whiskey. Mixtures are those composed of different compounds. Pure substances can be either chemical compounds or elements. Crystallization separates mixtures based on differences in solubility, a measure of how much solid substance remains dissolved in a given amount of a specified liquid. Mass is a fundamental property of an object that does not depend on its location. Open in App. It is uniform in composition throughout.
Fresh orange juice takes a little work, but it tastes much better than orange juice from concentrate. I came up with this recipe in response to a request about how to make fresh-squeezed orange juice.
Yes, orange juice is considered a suspension. However, they may still harbor additives. If you removed the pulp and other organic substances that are not dissolved, and processed the heck out of it, you could end up with something that qualifies as a homogeneous mixture. Gravity does not pull them down or out. It is composed of multiple substances that are not bonded to each other, making it a mixture. Is orange juice a solution? The reason? B Because its composition is not uniform throughout, orange juice is a heterogeneous mixture. Pure substances are either elements or compounds while a mixture is a combination of two or more products. When you squeeze an orange, the juice comes out.

0 thoughts on “Orange juice with pulp pure substance or mixture”