Number of structural isomers possible in c3h6o
Sign in Open App. Most Upvoted Answer.
Assertion : Carbon oxygen bond length of phenol is slightly less than that in methanol. Reason : There exist a partial double bond character and carbon to which oxygen is attached in phenol is s p 2 hybridised. An important route to unsymmetrical ethers is a nucleophilic substitution reaction known as the Williamson synthesis. This synthesis consists of an S N 2 reaction of a sodium alkoxide with an alkyl halide, alkyl sulphonate or alkyl sulphate. By a proper choice of reagents, both symmetrical and unsymmetrical ethers can be prepared by Williamson synthesis. The reverse process of cleavage of ethers to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at K. Which of the following reagents when heated will give a good yield of ether?
Number of structural isomers possible in c3h6o
.
In the given reactions, X and Y are :.
.
After completing this section, you should be able to explain the differences among constitutional structural isomers and stereoisomers geometric isomers. The following flow chart can be used to identify the relationship of two compounds with respect to isomerization:. Each sp 3 hybrid orbital is cylindrically symmetrical all cross-sections are circles , resulting in a carbon—carbon single bond that is also cylindrically symmetrical about the C—C axis. Because rotation about the carbon—carbon single bond can occur without changing the overlap of the sp 3 hybrid orbitals, there is no significant electronic energy barrier to rotation. Consequently, many different arrangements of the atoms are possible, each corresponding to different degrees of rotation. Unlike conformational isomers, which do not differ in connectivity, structural isomers differ in connectivity, as illustrated here for 1-propanol and 2-propanol. Although these two alcohols have the same molecular formula C 3 H 8 O , the position of the —OH group differs, which leads to differences in their physical and chemical properties.
Number of structural isomers possible in c3h6o
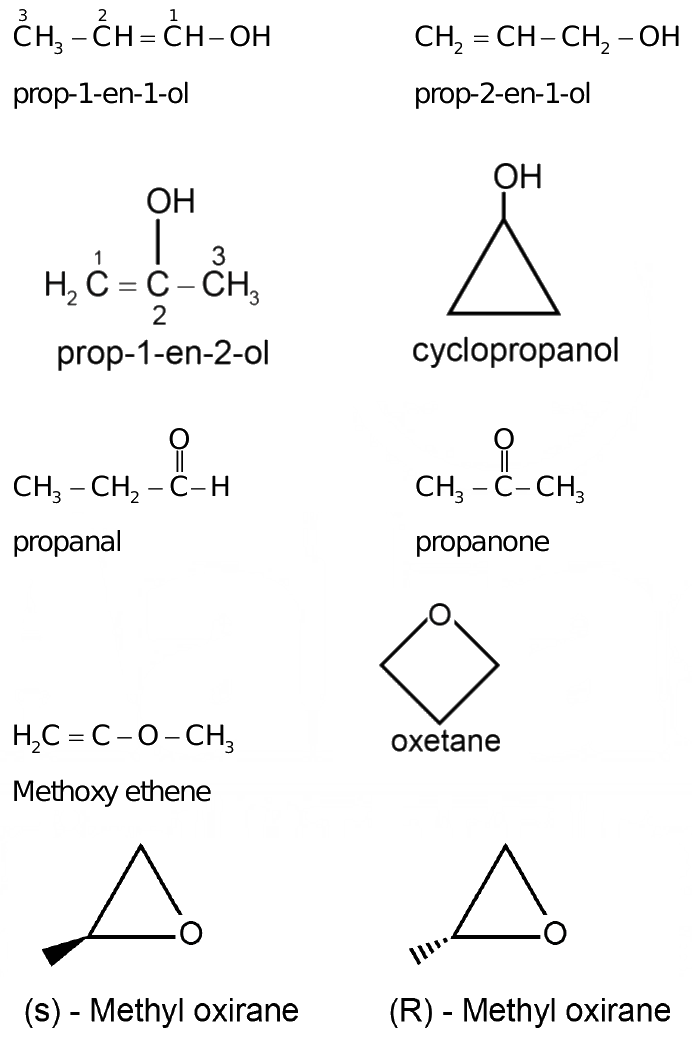
Sign in Open App. Most Upvoted Answer. Prop1en1ol, 2. Prop1en2ol, 3. Prop1en2ol, 4. Methoxyethene, 5.
Mozart k 488
View all answers. In this case, the carbon chain contains three carbon atoms, and the carbonyl group is attached to one of the end carbon atoms. Enter OTP. There are many different classes of isomers, like stereoisomers, enantiomers, and geometrical isomers. Solutions for No. Sign in Open App. How many structural isomers are possible in C3H7Cl? Information about No. Two or more compounds with the same formula but different arrangements of the atoms are called isomers. Copy Link.
For C 3 H 6 O chemical formula, we can draw different isomers. C 3 H 6 O is a chemical formula for several organic compounds.
For Your Perfect Score in Class Cyclopropanol, 8. View All Tests. Signup to see your scores go up within 7 days! Due to the presence of ambidentate ligands, coordination compounds show isomerism. Information about No. Here you can find the meaning of No. Select the incorrect statement regarding Kolbe's reaction. Copy Link. Similar Class 12 Doubts Read the passage given below and answer the following questions:The existence of coordination compounds with the same formula but different arrangements of the ligands was crucial in the development of coordination chemistry. The major product of the given reaction is- Explore Courses for Class 12 exam.

0 thoughts on “Number of structural isomers possible in c3h6o”