Noble gas configuration chart
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more noble gas configuration chart in their outer shells. They are completely full and cannot handle any more.
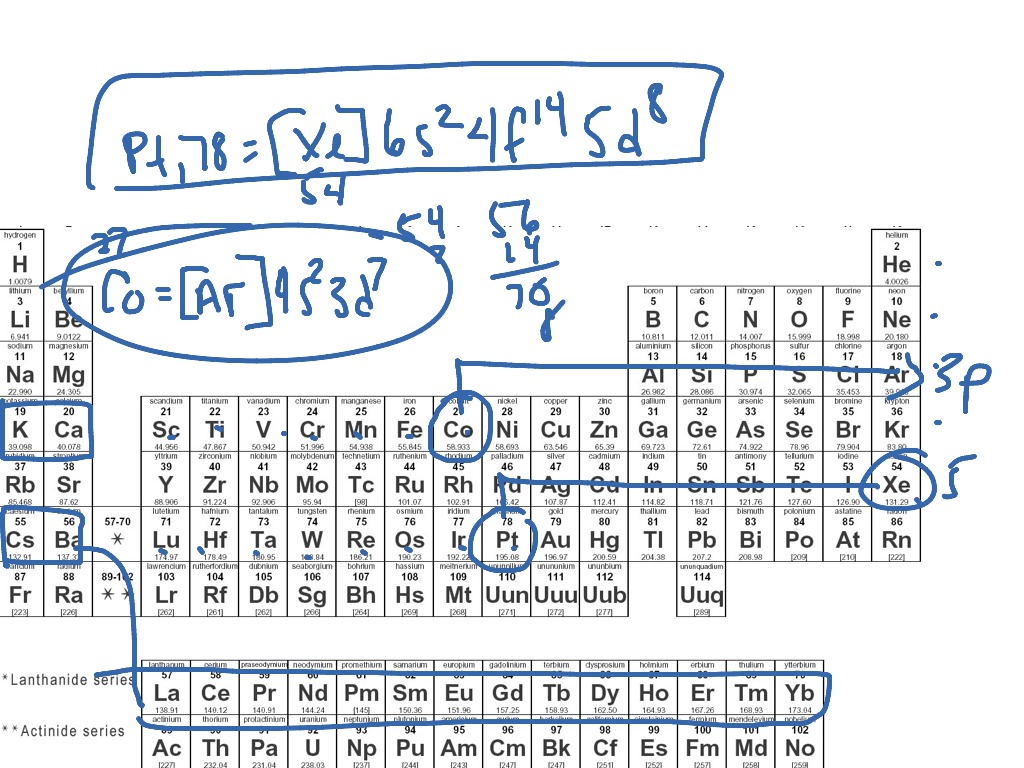
The content that follows is the substance of General Chemistry Lecture In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:. So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital.
Noble gas configuration chart
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times. Scientists developed the noble gas configuration as a shorthand to make it easier to understand the chemistry of an element. Skip to Content. Edit this Article. Popular Categories. Arts and Entertainment Artwork Books Movies. Relationships Dating Love Relationship Issues. Hobbies and Crafts Crafts Drawing Games. All Categories. Log in Social login does not work in incognito and private browsers.
For each tiktokcounter the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. You Might Also Like. All Categories.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus element 15 as an example, the concise form is [Ne] 3s 2 3p 3. Here [Ne] refers to the core electrons which are the same as for the element neon Ne , the last noble gas before phosphorus in the periodic table. The valence electrons here 3s 2 3p 3 are written explicitly for all atoms.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. To save room, the configurations are in noble gas shorthand. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. Look up the electronic configuration of that noble gas and include that value before the rest of the configuration. This table is available to download as a PDF to use as a study sheet. Values denoted by an asterisk are predictions based on periodic table trends.
Noble gas configuration chart
As you have learned, the electron configurations of the elements explain the otherwise peculiar shape of the periodic table. Although the table was originally organized on the basis of physical and chemical similarities between the elements within groups, these similarities are ultimately attributable to orbital energy levels and the Pauli principle, which cause the individual subshells to be filled in a particular order. For example, the two columns on the left, known as the s block , consist of elements in which the ns orbitals are being filled. The six columns on the right, elements in which the np orbitals are being filled, constitute the p block. Within each column, each element has the same valence electron configuration—for example, ns 1 group 1 or ns 2 np 1 group As you will see, this is reflected in important similarities in the chemical reactivity and the bonding for the elements in each column. Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 columns in the d block, and 14 columns in the f block. Hydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1 s 1 electron configuration suggests a similarity to lithium [He]2 s 1 and the other elements in the first column.
Navel brunch & coffee
Toggle limited content width. But again the construction of the electron configuration gives us the answer. In these cases, you can use the previous noble gas to abbreviate the configuration as shown below. This should be intuitive since with each row of the table you are adding a shell n. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. The fourth electron shell has an s, p, d, and f energy level. What is not changing as you cross a period? From these electronegativity values we can derive the patterns of two other periodic properties: Ionization Energy and Electron Affinity. Aqueous chemistry Crystal structure Electron configuration Electronegativity Goldschmidt classification Term symbol.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals.
Periodic table electron configurations Electron configurations of the elements data page. Part 1. That leaves 5 electrons. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. From these electronegativity values we can derive the patterns of two other periodic properties: Ionization Energy and Electron Affinity. So think of it this way, the inner shell electrons are a shield against the pull of the nucleus. With 10 electrons you should note that oxygen's electron configuration is now exactly the same as Neon's. Submit a Tip All tip submissions are carefully reviewed before being published. This article has been viewed , times. Summary The noble gas configuration system allows some shortening of the total electron configuration by using the symbol for the noble gas of the previous period as part of the pattern of electrons. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. Not Helpful 7 Helpful Following the 2s sublevel is the 2p, and p sublevels always consist of three orbitals.

0 thoughts on “Noble gas configuration chart”