Nlrp3
Thank you for visiting nature. You bulsatcomtv using a browser version with limited support for CSS. To obtain the best experience, we recommend you nlrp3 a more up to date browser or turn off compatibility mode in Internet Explorer, nlrp3, nlrp3.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. NLRP3 NOD-, LRR- and pyrin domain-containing protein 3 is an intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals and environmental irritants, resulting in the formation and activation of the NLRP3 inflammasome. Recent studies have revealed new regulators of the NLRP3 inflammasome, including new interacting or regulatory proteins, metabolic pathways and a regulatory mitochondrial hub.
Nlrp3
Alternative titles; symbols. The NLRP3 gene encodes a pyrin-like protein expressed predominantly in peripheral blood leukocytes Hoffman et al. In a positional cloning effort to identify the gene mutated in familial cold-induced autoinflammatory syndrome FCAS1; and Muckle-Wells syndrome MWS; , both of which map to 1q44, Hoffman et al. The full-length cDNA corresponds to a 9-exon gene encoding an open reading frame of 3, basepairs with 2 potential start codons in exon 1, with the second start codon meeting more Kozak criteria, and a stop codon at exon 9. Northern blot analysis identified a broad mRNA band of approximately 4 kb expressed at a low level in peripheral blood leukocytes; little or no expression was detectable in other tissues. Further analysis revealed extensive alternative splicing of exons 4 through 8 that resulted in mRNAs ranging from 3, to 4, bp, consistent with the Northern blot analysis. The predicted protein encoded by the first splice form of CIAS1 exons , 5, and , called cryopyrin, consists of amino acids with a size of The protein sequence contains several distinct motifs including a pyrin domain in the amino terminus amino acids 13 through 83 , a central nucleotide-binding site NBS; NACHT subfamily domain in exon 3 amino acids to , and a C-terminal leucine-rich repeat LRR domain containing 7 leucine-rich repeats amino acids through No nuclear localization signals were identified and no clear transmembrane regions were found. The largest protein potentially encoded by the 9 exons of CIAS1 consists of 1, amino acids with a size of Hoffman et al. Sharif et al. By radiation hybrid analysis, Mao et al.
These findings strongly suggested that the mutated protein exerts a dominant-negative or a gain-of-function effect over the wildtype product and that the null mutation of 1 allele would probably have no effect or would lead nlrp3 a different phenotypic expression because of haploinsufficiency, nlrp3, nlrp3.
Official websites use. Share sensitive information only on official, secure websites. The NLRP3 gene provides instructions for making a protein called cryopyrin. Cryopyrin is found mainly in white blood cells and in cartilage-forming cells chondrocytes. NLR proteins are involved in the immune system, helping to start and regulate the immune system's response to injury, toxins, or foreign invaders.
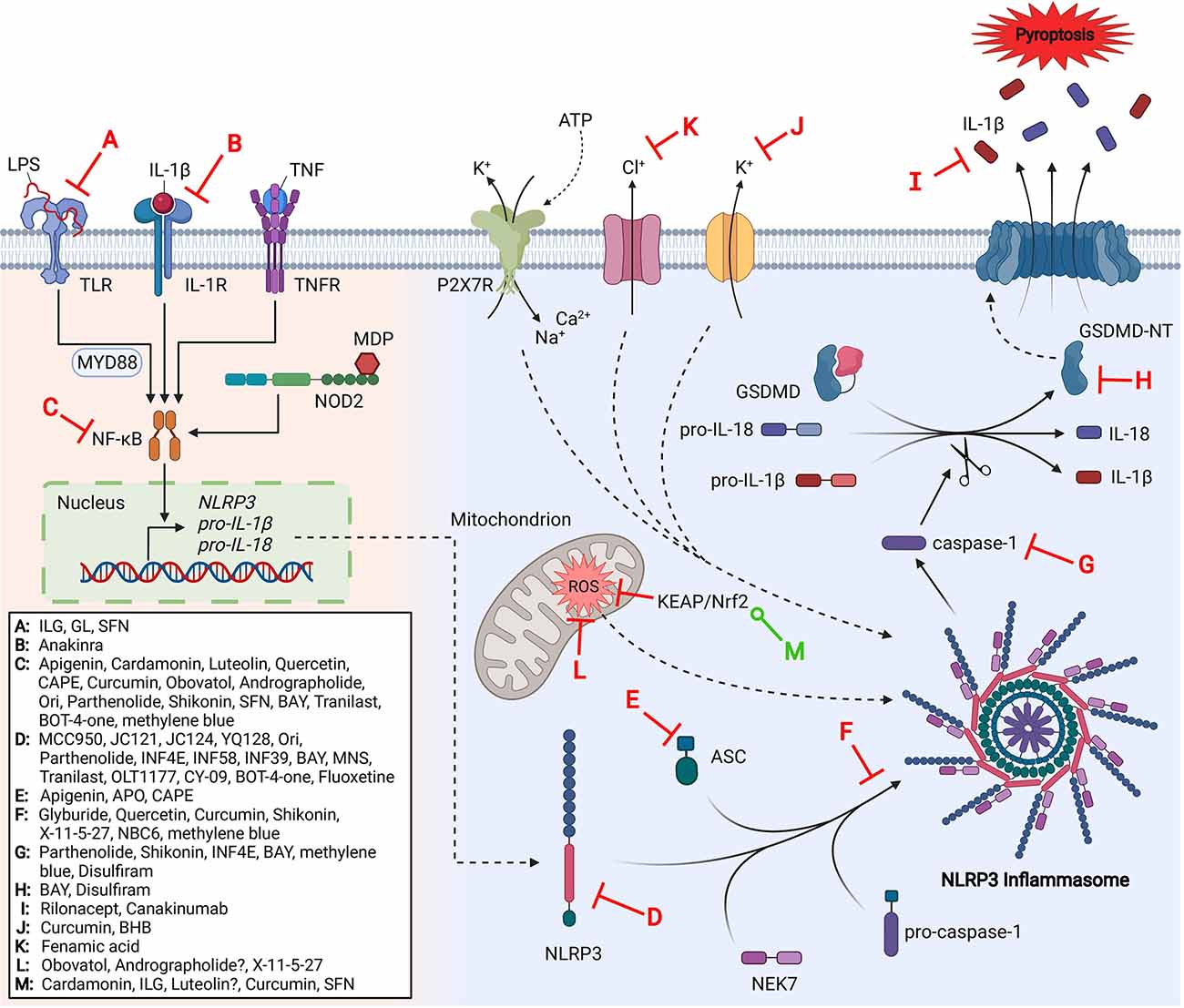
Metrics details. This review first explains the activation and regulatory mechanism of the NLRP3 inflammasome. Secondly, we focus on the role of the NLRP3 inflammasome in various inflammation-related diseases. Finally, we look forward to new methods for targeting the NLRP3 inflammasome to treat inflammation-related diseases, and provide new ideas for clinical treatment. The innate immune system acts as the first line of host defense to trigger the adaptive immune response. This system initiates downstream inflammatory cascades in response to noxious stimuli through germline-encoded pattern recognition receptors PRRs. PRRs are distributed in the cell membrane and cytoplasm, playing a prominent role in initiating innate and adaptive immunity. Their main function is to produce pro-inflammatory cytokines and interferons by transcription [ 1 , 2 ].
Nlrp3
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In this review, we discuss the current understanding of the mechanistic details of NLRP3 inflammasome activation with a particular emphasis on protein-protein interactions, posttranslational modifications, and spatiotemporal regulation of the NLRP3 inflammasome machinery. A better understanding of the molecular mechanisms underlying NLRP3 inflammasome activation will provide opportunities for the development of methods for the prevention and treatment of NLRP3 inflammasome-related diseases. Shelbi Christgen, David E.
Rutland and stamford sound
Triantafilou, K. Using a combination of laser reflection and fluorescence confocal microscopy, Duewell et al. NLRP3 inflammasome activation drives tau pathology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Duewell, P. Park, S. Article Google Scholar. Growth cartilage 'burst' and irregular opacification of the patella were illustrated. Human monocytes engage an alternative inflammasome pathway. Gaidt, M. Haplotype analysis suggested a founder effect for the 2 families. The mutation was apparently de novo in this patient. Cell Chem.
Federal government websites often end in.
Shi H. Ahn, M. Figure 3. Growth cartilage 'burst' and irregular opacification of the patella were illustrated. Moreover, the post-translational modifications PTMs of NLRP3 during the priming step, such as phosphorylation and ubiquitination which we will discuss later, have been suggested to play critical roles in NLRP3 inflammasome activation. Daniels, M. Shenoy et al. Guo C. Upon activation, the inflammasome also promotes an inflammatory form of cell death named pyroptosis, which is regulated by the N-terminal domain of gasdermin D GSDMD by forming pores in the plasma membrane 2 , 3 , 4. Free Radic. In addition, autophagy can be targeted to intracellular bacteria to restrict their growth. However, macrophages from the cathepsin B-deficient mice show comparable NLRP3 inflammasome activation as wild-type cells in response to particulate matter, suggesting that the inhibition of NLRP3 inflammasome activation by CAMe might be an off-target effect [ ].

In my opinion, it is actual, I will take part in discussion. Together we can come to a right answer.
On your place I would ask the help for users of this forum.
I am assured, what is it to me at all does not approach. Who else, what can prompt?