Nitrogen trifluoride lewis structure
The NF3 molecule, composed of one nitrogen atom and three fluorine atoms, nitrogen trifluoride lewis structure, holds within its structure a fascinating arrangement of atoms and electrons that govern its chemical behavior. By delving into the principles of valence electrons, formal charges, and the octet rule, we can decipher the molecular puzzle that NF3 presents.
Nitrogen trifluoride NF3 is a colorless, nonflammable gas with routine usage in the microelectronics industry. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, and its heat storage capacity is 17, times that of carbon dioxide. The molecule is a hazardous greenhouse gas that can persist in the atmosphere for years. In , it was included in the list of controlled gases under the United Nations Framework Convention on Climate Change[]. Fluorine is a group VIIA element and has seven electrons in its last shell valence shell. Nitrogen is a group VA element in the periodic table and contains five electrons in its last shell.
Nitrogen trifluoride lewis structure
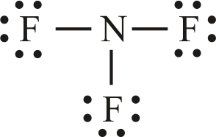
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. You have to follow few steps to draw the lewis structure of NF 3. Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps which are used to draw lewis structures of complex molecules and ions. Those steps are explained in detail in next sections. There are two elements in NF 3 ; fluorine and nitrogen. Fluorine is a group VIIA element and has seven electrons in its last shell valence shell.
Now, we know how many electrons includes in valence shells of fluorine and nitrogen atoms. Each fluorine atom shares one electron with the nitrogen atom, resulting in three covalent bonds in the NF3 molecule.
The Lewis structure of nitrogen trifluoride NF 3 consists of three N-F bonds and a lone pair on the nitrogen atom, while each fluorine atom has three lone pairs. Drawing the Lewis structure of NF 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. The Lewis structure of NF 3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs. In the Lewis structure of the NF 3 molecule, a central nitrogen atom is surrounded by three fluorine atoms. Each nitrogen — fluorine bond is represented by a single line, and there is a lone pair of electrons on the nitrogen atom.
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule.
Nitrogen trifluoride lewis structure
Nitrogen trifluoride NF 3 is an inorganic, colourless, non-flammable, toxic gas with a slightly musty odour. In the NF 3 molecule, nitrogen is attached to three fluorine atoms via a single bond and has a molecular weight of Before arranging the atoms, one should know which atom will occupy the central position. As per the electronegativity rule, the atom with a less electronegative nature will take that position. Hence, Nitrogen will place at the center, and the rest of the atoms will take peripheral positions. NF 3 lewis structure.
Hulk nutrition
As a result, the fluorine atoms pull the electrons in the NF3 molecule closer to themselves, creating an uneven distribution of electron density. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Once the Lewis structure is drawn, the shape of the NF 3 molecule can be determined. In the case of NF 3 , the total number of valence electrons is 26 , resulting in a total of 13 valence electron pairs. This indicates that Nitrogen and fluorine are chemically bonded with each other in an NF3 molecule. Remember that, there are total of thirteen electron pairs. Step by step drawing the Lewis structure of NF 3 To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. Nitrogen trifluoride is a chemical compound with chemical formula NF3. Begin by identifying the number of valence electrons for each atom in the NF3 molecule. So, we have successfully obtained the Lewis structure of NF 3. Finally, the remaining lone pair can be marked on the nitrogen atom.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
Finally, the remaining lone pair can be marked on the nitrogen atom. In the NF3 molecule, put the electron pairs between the nitrogen atom N and fluorine atoms F. Each fluorine F atom now has 8 electrons 2 in the bond and 6 in lone pairs , making them stable. The final Lewis structure for NF3 is as follows:. Lewis structure of nitrogen trifluoride NF 3 The Lewis structure of NF 3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs. Place the remaining 20 valence electrons around the atoms as lone pairs. In the NF3 Lewis structure, lone pairs are placed on atoms to fulfill the octet rule and achieve electron stability. How many lone pairs of electrons are on nitrogen in nf3? Step by step drawing the Lewis structure of NF 3 To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. Place two lone pairs 4 electrons on the nitrogen atom. Since the overall formal charge is zero, the above Lewis structure of NF3 is most appropriate, reliable, and stable. You have to follow few steps to draw the lewis structure of NF 3. Therefore, we do not need to worry about reducing charges on atoms to get the best stable structure. For, NF 3 S, Total pairs of electrons are thirteen. Therefore, three lone pairs will be marked on each fluorine atom, totaling 9 electron pairs.

No doubt.