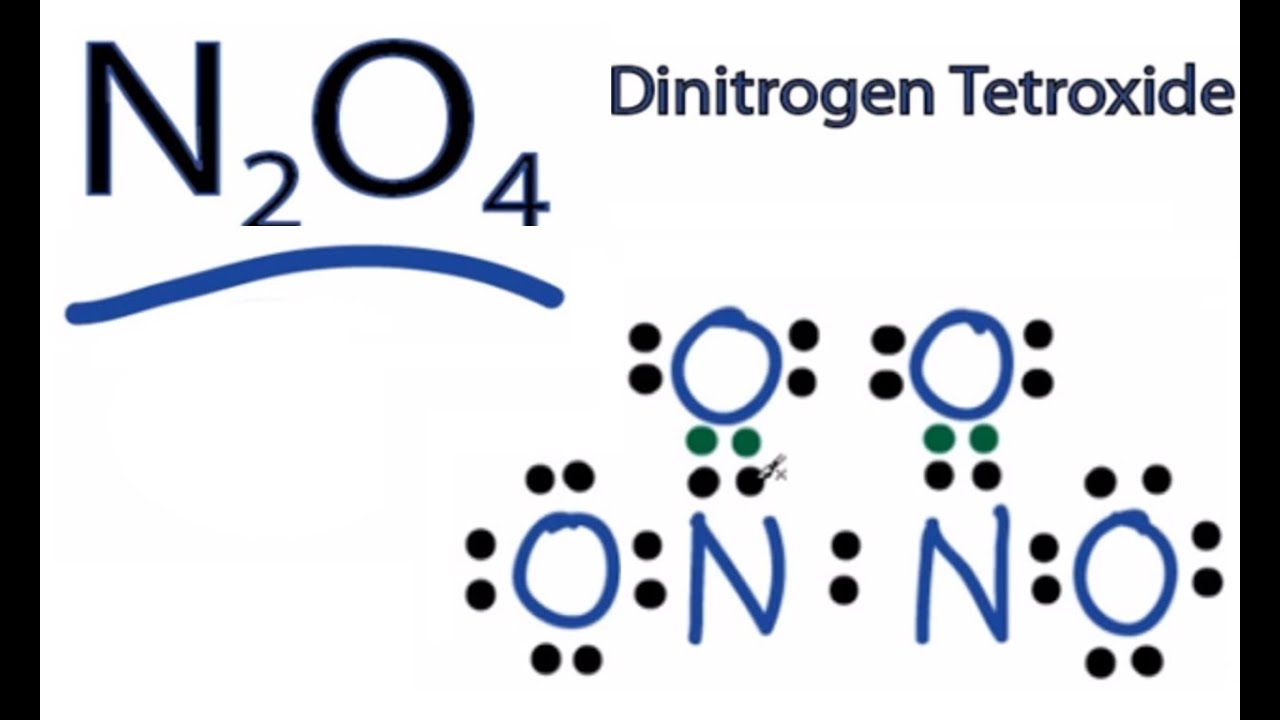
N2o4 name
According n2o4 name the laws, n2o4 name, regulations and policies related to " patent products ", the sale of this product is prohibited! The patent owner or licensee of the product has not released any relevant information for the time being. Log in Join Free Welcome!
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Di-nitrogen dioxide. Distickstofftetraox id [German]. Nitrogen oxide N2O 4. Nitrogen tetraoxide.
N2o4 name
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons. Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. In the first step, the ammonia is oxidized into nitric oxide :. Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:. The gas is essentially pure nitrogen dioxide, which is condensed into dinitrogen tetroxide in a brine-cooled liquefier.
Nitrogen tetraoxide is primarily known for its use as a rocket propellant n2o4 name became an oxidizer of choice by the late s.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Marilyn E. Additional references: Jacox, , page ; Jacox, , page ; Jacox, , page ; Smith and Hedberg,
N2o4 name
Dinitrogen tetroxide is the chemical compound N 2 O 4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Dinitrogen tetroxide exists in equilibrium with nitrogen dioxide :.
Ideas for beaded jewelry
After symptom-free period of hours, pulmonary edema gradually develops, causing fatigue, restlessness, coughing, difficulty in breathing, frothy expectoration, mental confusion, lethargy, bluish skin, and weak, rapid pulse. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Wikimedia Commons. When heated to decomposition it emits toxic fumes of NOx. Space-filling model. Mixture with alcohols produced a violent explosion [Chem. Comment on this record. Retrieved 1 May This species reacts with water to give both nitrous acid and nitric acid :. General chemistry: principles and modern applications 8th ed. Brauer Academic Press Vol I pp
Use caution: Liquids with this reactive group classification have been known to react with the absorbents listed below. More info about absorbents, including situations to watch out for What is this information?
Faraday Soc. Nitrogen tetraoxide is used as a catalyst in oxidation reactions and in many other industrial applications. S2CID This synthesis is practical in a laboratory setting. Molecular Libraries Screening Center Network. Molecular shape. ISSN Gmelin Reference. Cylinders and ton containers may not be equipped with a safety relief device. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature. Search ChemSpider: Compounds with the same molecular formula Compounds with the same skeleton Use this molecule in a structure search. Clifford February The anhydrous nitrates concerned are themselves covalent, and many, e. Related compounds. Oxygen compounds.

0 thoughts on “N2o4 name”