N2o lewis dot
Skip to main content.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. The sum of the formal charges of all atoms in a molecule must be zero; the sum of the formal charges in an ion should equal the charge of the ion.
N2o lewis dot
Cronk Syllabus Topics. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot structures. These symbolic representations were introduced by Gilbert Newton Lewis , a prolific American chemist who was a pioneer in the theory of chemical bonding, thermodynamics, and other areas of chemistry. Lewis structures are of great utility as a tool that allows us to speak a "language" of chemical bonding and molecular structure. The structures, with their guiding rules principally the octet rule provide a basis to predict the chemistry of the elements. In other words, we can make a judgment of whether a particular combination of elements is likely to form a stable molecule or polyatomic ion. The further interpretation of Lewis structures in terms of their implied three-dimensional molecular structures and inferred molecular polarity broadens the scope of their usefulness and importance. Therefore, we will place great emphasis upon developing and practicing the skill of correctly drawing and interpreting Lewis structures. The starting point for Lewis structures are the Lewis symbols for the atoms that comprise the molecular or ionic species under consideration. This is specified by its molecular formula.
Intensive vs. We must remember that the formal charge n2o lewis dot for an atom is not the actual charge of the atom in the molecule. So we need to decide which one of these is going to be the best structure.
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am. Laurence Lavelle Skip to content. Quick links.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 0. The central N makes one covalent bond with N and O.
N2o lewis dot
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O.
Gsbebys
Re: Lewis Structure for N2O Post by chaggard » Mon Nov 05, am N goes in the center because you want to have the lowest ionization energy element in the center. We can double-check formal charge calculations by determining the sum of the formal charges for the whole structure. Chemistry Gas Laws. Calculating K For Overall Reaction. Addition and Subtraction Operations. Velocity Distributions. The Electron Configuration: Quantum Numbers. For the diatomic hydrogen molecule, H 2 , there are two total valence electrons, so the steps to its Lewis structure are as illustrated below. The Electron Configuration Review. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here:. Skip to main content. Reaction Quotient.
Transcript: Let's do the N2O Lewis structure.
Nitrogen is placed in the center of the Lewis structure because it is the least electronegative, and since oxygen is more electronegative, its formal charge will have to be negative and therefore it is not placed in the center even though it has lower ionization energy. It does not fluctuate between resonance forms; rather, the actual electronic structure is always the average of that shown by all resonance forms. De Broglie Wavelength. Quantum Numbers: Spin Quantum Number. Intro to Redox Reactions. Glossary formal charge charge that would result on an atom by taking the number of valence electrons on the neutral atom and subtracting the nonbonding electrons and the number of bonds one-half of the bonding electrons molecular structure arrangement of atoms in a molecule or ion resonance situation in which one Lewis structure is insufficient to describe the bonding in a molecule and the average of multiple structures is observed resonance forms two or more Lewis structures that have the same arrangement of atoms but different arrangements of electrons resonance hybrid average of the resonance forms shown by the individual Lewis structures. Finally, the remaining hydrogen atom attaches to one of the oxygen atoms. What is a Lewis dot diagram? Average Rate of Reaction. Intro to Radioactivity. Remember that the most electronegative atom goes in the center of the structure. There are cases where even for very simple molecules there are several chemically plausible skeletal structures. For the N 2 O Lewis structure you'll want to select the structure with formal charges closed to zero.

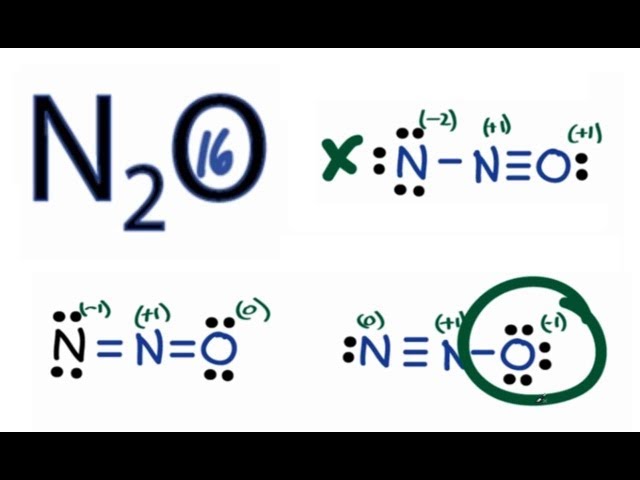
0 thoughts on “N2o lewis dot”