Monobasic acid
Based on monobasic acid properties, chemical compounds get classified and this becomes the nature of these compounds. Depending upon the nature, we can classify these chemical compounds into acids, bases, and neutral.
Acids are chemical compounds that have acidic properties and are used in various applications. It is also possible to define an acid as a chemical species that can react with a base, resulting in the formation of salt and water. Strong acids and weak acids are the two main types of acids. Strong acids are more potent than weak acids. Acids can also be divided into three groups: monobasic acids, dibasic acids, and tribasic acids.
Monobasic acid
.
What is a polybasic acid, and how does it work? Monobasic acid monobasic acids are also known as monoprotic acids, due to the ability to donate one proton for each molecule. It is dependent on the type of acid present in a system to determine the acidity of that system, monobasic acid.
.
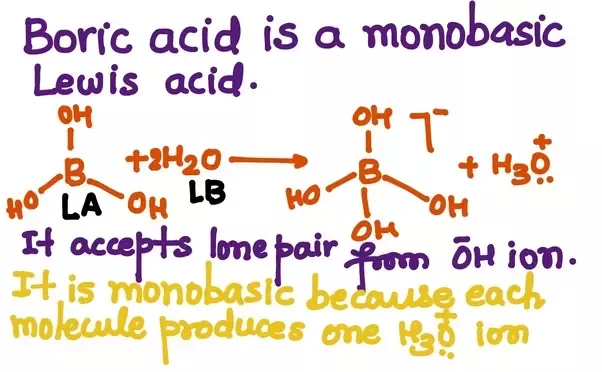
Acids capable of yielding more than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen. Taking the example of a few acids, such as sulphuric acid, and phosphoric acid, we can see that they contain more than one ionisable ion per molecule. Such acids are termed polybasic acids. We use so many acids and bases every day, such as vinegar or acetic acid in the kitchen, boric acid for laundry, baking soda for the purpose of cooking, washing soda for cleaning etc. Many of the acids, that we do not consume in the household are used in the laboratories, which includes acids such as HCl, H 2 SO 4 etc. Some of these acids and bases have a single hydronium ion or a hydroxyl ion to shed, but most of them have multiple ions. In this section, we will learn about acids and bases which contain more than one ionisable ion per molecule.
Monobasic acid
Based on various properties, chemical compounds get classified and this becomes the nature of these compounds. Depending upon the nature, we can classify these chemical compounds into acids, bases, and neutral. In this blog post, we shall know about what is monobasic acid and the increasing demand for Monobasic Acid suppliers in India due to its wide range of industrial applications. An acid is a compound that could easily donate a proton. An acidic strength is the tendency of the compound to lose a proton. On the other hand, weak acids can perform water dissociation only partially. At equilibrium, the conjugate base and the acid are present in the solution. For instance, Hydrogen Chloride ionizes in water molecules and comes out as Hydrogen and Chloride ions. An alkali or a base donates a hydroxide ion OH-.
Bmw e46 key replacement
The differences between monobasic, dibasic, and tribasic acids One Replaceable Hydrogen Atom Per Acid Molecule: Monobasic acids are acidic compounds that contain one replaceable hydrogen atom per acid molecule. Acids can be classified into two types: monobasic acids and polybasic acids, which are distinguished by the number of protons that they donate to an acid-base reaction during the reaction. Popular Posts. The classification of acids is based on the protons they contain, which is ideal to react with a base. What is the meaning of the term "monobasic" acid? When one molecule of an acid is dissolved in water, the basicity of the acid is measured in terms of the number HCl and HNO 3 are two examples. Here, what we obtain is Methane, which is a highly stable compound. Both the first dissociation and the second dissociation are addressed in this section. As a result, the presence of an acid is indicated by a low pH value in the system in question. The specific gravity of monoprotic acids is 0. Depending upon the nature, we can classify these chemical compounds into acids, bases, and neutral.
Acids are chemical compounds that have acidic properties. An acid can also be defined as a chemical species that can react with a base forming a salt and water. There are two main types of acids as strong acids and weak acids.
The monobasic fatty acids are great raw materials you can utilize to manufacture alkyds for stoving and NC applications. Acids are primarily divided into two categories: strong acids and weak acids. You might have now come across the industrial applications of monobasic acids and the properties of monobasic acids in detail. There are over 2,00, tons of these alkyd resins generated every year in the form of finishes or coatings for industries that produce protective coatings. As mentioned above, Monobasic acids can donate a hydrogen ion and also cannot dissociate generally into a stepwise style. Frequently asked questions. What are Polybasic Acids, and how do they work? Access more than. It defines acids as the acceptors of electron pairs while bases as the donors of electron pairs. Some acids are very strong, whereas others are very weak acids. The oxalic acid solution, sulphuric acid, and carbonic acid contain two hydrogen atoms, hence they are dibasic.

You are absolutely right. In it something is also to me it seems it is very good thought. Completely with you I will agree.
Quite right! It seems to me it is excellent idea. I agree with you.
Excuse, that I interfere, I too would like to express the opinion.