Moles to moles calculator
The calculator below calculates the mass of the substance in grams or the quantity of the substance in moles.
We've updated our Privacy Policy to make it clearer how we use your personal data. We use cookies to provide you with a better experience. You can read our Cookie Policy here. Molarity is the concentration of a solution in terms of the number of moles of the solute in 1 dm 3 1 liter of the solution. A mole is the quantity of anything that has the same number of particles as 12 g of carbon
Moles to moles calculator
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator? Well, then, you've come to the right place. With our moles-to-grams converter, you can seamlessly convert between mass, molecular weight, and moles. Chemistry just became that little bit easier! Impress your friends with your astounding ability to find how many moles of a substance you have at a kilogram, ounce, or even tonne scale! Also useful for any serious industrial applications, for all you chemical engineers out there. While we're on the topic of chemistry, we have several other calculators that you might find useful. Why not check out our molarity calculator or our percent yield calculator? A mole is a small, subterranean mammal belonging to the family Talpidae. Just kidding — we're sure you've never heard that joke before. A mole is how chemists define an amount of substance, useful when dealing with many different molecules reacting at once i.
You can read our Cookie Policy here. In many chemistry problems, you need to convert grams to moles or moles to grams.
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio? Join us in this article as we discuss the definition of a molar ratio, how to determine it using the molar ratio formula, and how to use it to get more information from your balanced chemical equation. The molar ratio is different from a mole fraction.
Moles for chemists are not only found in gardens but appear everywhere: our mole calculator teaches you everything you need to know about this concept. A mole is a fundamental quantity in chemistry, slightly challenging our understanding but of utmost importance in every aspect of that science — and not only. And much more: keep reading to dive deep into the fundamental aspects of chemistry. Are you also interested in biology? Be sure to visit our protein molecular weight calculator , where you can learn a lot about the molecular weight of organic materials. We will introduce the concept of mole and gradually explain all the intricacies associated with it. A mole is a quantity of a substance that contains exactly an Avogadro's number of molecules or atoms. The Avogadro's number is a physical constant hence it has an exact and fixed value :. Why does Avogadro's number have this value?
Moles to moles calculator
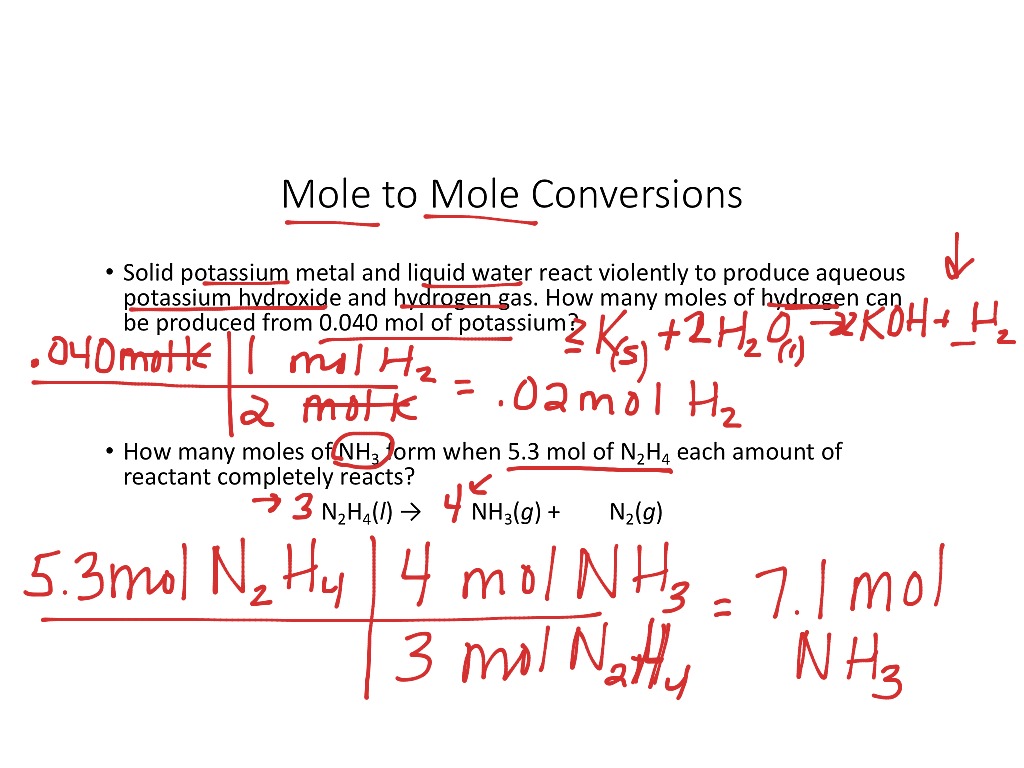
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as.
Napa valley paddle
We know we have 10 g of HCl, which has a molecular weight of Molecular mass also called molecular weight - sum of the atomic weights of all atoms appearing in a given molecular formula. Make an informed choice with our handy tool. Since each mole of oxygen produces twice as many moles of water, it makes sense that the produced amount is greater than the reactant amount. Books vs e-books Calculator Discover the ultimate paper books vs. Calculate Molarity? Find a balanced equation that describes the reaction. Rearranging the equation is not necessary as the calculator tool will do this for you. Well, as we said above, it provides a useful metric when dealing with reactions. It is possible to calculate the mass of each reactant and product required if you know the molar ratio , number of moles required, and molecular weight of each reactant and product. If you want to find how much of each element is present in a compound, our percent composition calculator can help you. The equation is also balanced if we were to write it as. Calculate Number of Moles? Therefore, you need to be able to convert from weight and volume to moles and vice versa to conduct experiments in a laboratory or carry out reactions to manufacture products in industry. If you wanted to find the concentration of the hydrochloric acid, you could use our concentration calculator.
Please enable Javascript to use the unit converter. How many moles in 1 mole?
For example, we've seen that the molar ratio equation between hydrogen and oxygen in water production is To calculate the molar ratio from the mass of the reactants or products , follow these simple steps:. As follows, we will extend the meaning of the coefficients in a chemical equation. The molar mass is the mass in grams of 1 mole of a particular molecule. We can leave out the word mol and not write the 1 coefficient as is our habit , so the final form of the equation, still balanced, is. It also displays the molar mass of the chemical substance and details of its calculation just for reference. To convert grams to moles: Find a periodic table. People also viewed…. Since the molar ratio helps determine how much of each substance is required to complete the reaction, you can also use it to find out which substance is the limiting reagent and which substance is in excess. You can calculate molar mass by identifying the atoms in the molecular formula, then adding the atomic mass of each element together to find the molecular weight. To produce Find out the molar mass of the substance hint: you can use Molar mass of the substance alone to calculate molar mass. The formula for converting molecules to moles is a little different than mass and volume because both molecules and moles are measures of quantity:.

0 thoughts on “Moles to moles calculator”