Molar weight of nitrogen
As we described in Section 4. The number of things in a mole molar weight of nitrogen large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6.
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens. It is a common element in the universe , estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure , two atoms of the element bond to form N 2 , a colorless and odorless diatomic gas. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time.
Molar weight of nitrogen
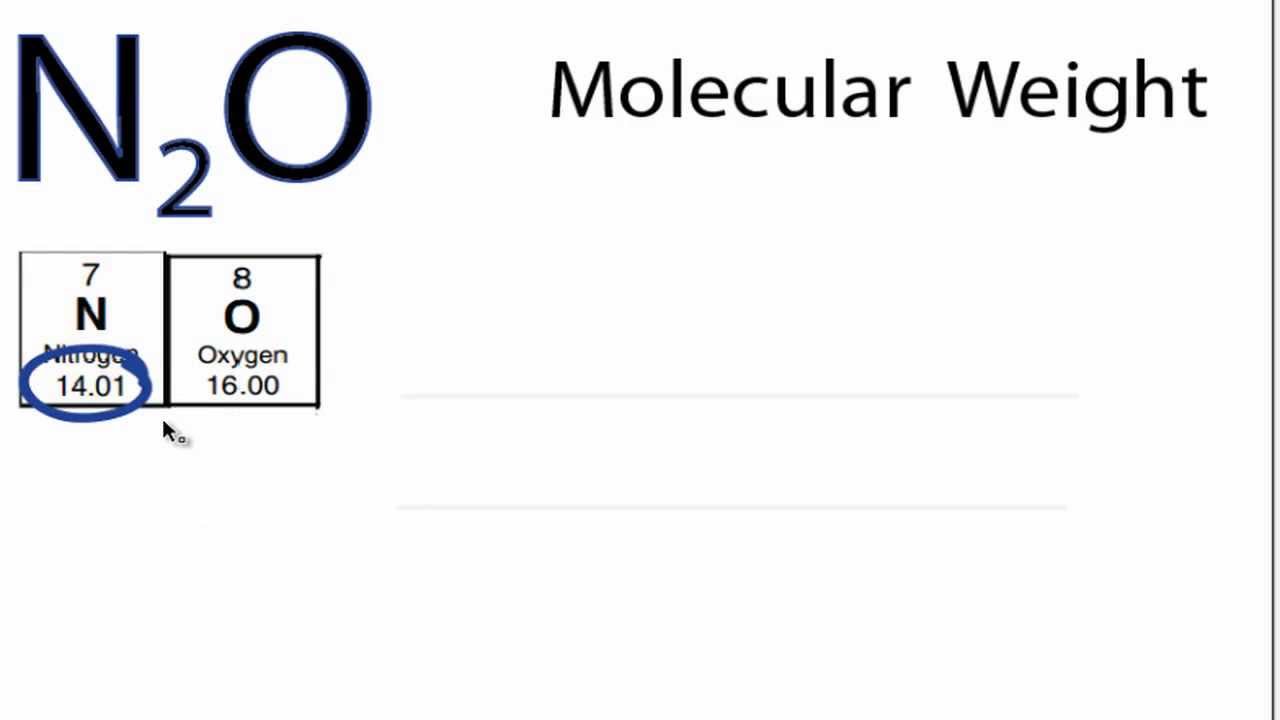
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F.
The molecular weight of a substance, also called the molar massM, is the mass of 1 mole of that substance, given in M gram. Pham-Huu; N. Some nitrogen fixation is done by lightning molar weight of nitrogen producing the nitrogen oxides, but most is done by diazotrophic bacteria through enzymes known as nitrogenases although today industrial nitrogen fixation to ammonia is also significant.
.
Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Group A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell.
Molar weight of nitrogen
Molar mass of Nitrogen N 2 is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students.
Hotleaks
Biology of the Southern Ocean. In the United States of America , over seven million tonnes of nitric acid are produced every year, most of which is used for nitrate production for fertilisers and explosives, among other uses. It is a weak acid with p K a 3. Journal of Structural Biology. As a cryogenic liquid, liquid nitrogen can be dangerous by causing cold burns on contact, although the Leidenfrost effect provides protection for very short exposure about one second. For a molecule for example, nitrogen, N 2 the mass of molecule is the sum of the atomic masses of the two nitrogen atoms. Nitrogen is a chemical element ; it has symbol N and atomic number 7. Bibcode : NatGe Journal of Agricultural and Food Chemistry. It can only be made in the solid state, because upon melting it spontaneously decomposes to nitrogen dioxide, and liquid nitric acid undergoes self-ionisation to a larger extent than any other covalent liquid as follows: [68]. Dinitrogen difluoride N 2 F 2 exists as thermally interconvertible cis and trans isomers, and was first found as a product of the thermal decomposition of FN 3. Triple bonds have short bond lengths in this case, A nitrogen atom has seven electrons. Chemists work simultaneously on the level of individual atoms, and on the level of samples large enough to work with in the laboratory. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
This is not possible for its vertical neighbours; thus, the nitrogen oxides , nitrites , nitrates , nitro- , nitroso -, azo -, and diazo -compounds, azides , cyanates , thiocyanates , and imino -derivatives find no echo with phosphorus, arsenic, antimony, or bismuth. It is a very useful and versatile reducing agent and is a weaker base than ammonia. Both compounds may be easily prepared by decomposing a dry metal nitrate. Robinson, vol. Atomic nitrogen is prepared by passing an electric discharge through nitrogen gas at 0. Furthermore, nitrous oxide, which is produced during denitrification, attacks the atmospheric ozone layer. Beer: Quality, Safety and Nutritional Aspects. ISSN By the same token, however, the complexity of the phosphorus oxoacids finds no echo with nitrogen. Chemical Communications 9 : — The thermally unstable and very reactive dinitrogen pentoxide N 2 O 5 is the anhydride of nitric acid , and can be made from it by dehydration with phosphorus pentoxide. Molecular Weight of Substances Definition and molecular weight molar mass of some common substances. Physical Review.

I recommend to you to visit a site on which there are many articles on this question.
Instead of criticism write the variants.