Molar mass of silver
Wiki User. In terms of metric conversion, mg of salt is equal to 0.
The silver molecule consists of 1 Hydrogen atom s - a total of 2 atom s. The molecular weight of silver is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass of each element. The atomic mass takes into account the isotopic distribution of the element in a given sample. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4.
Molar mass of silver
To solve this problem, you must find a conversion factor that can take you from moles of silver to grams of silver. To find that conversion factor, grab a periodic table and look for silver, "Ag". You'll find the element in period 5, group Your conversion factor is called molar mass and is located at the bottom of the block. The molar mass of an element tells you how the mass of exactly one mole of that element. In your case, silver is said to have a molar mass of " This tells you that every mole of silver has a mass of " If that is the case, then 7. You need to round this off to three sig figs , the number of sig figs you have for the number of moles of silver. Therefore, " g" of silver will contain 7. Stefan V. May 23, Explanation: To solve this problem, you must find a conversion factor that can take you from moles of silver to grams of silver.
Help is available! Please double check the email address It may or may not be correct. Online Unit Converters Miscellaneous Converters.
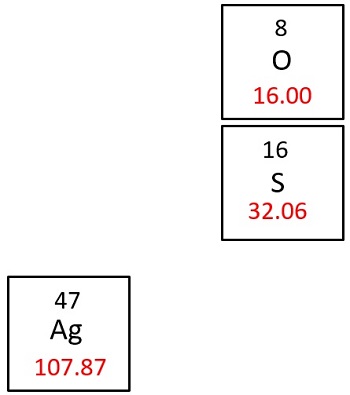
Molar mass of Ag 2 SO 3 Silver sulfite is Then, lookup atomic weights for each element in periodic table : Ag: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Our molar mass calculator comes to the rescue if you need to quickly check the weight of 1 mole of any element or chemical compound and you are unable to use the periodic table. Simply select one by one the elements from the list and give the number of atoms in their molecular formula to get the molar mass in a flash. Many similar tools are case-sensitive, so you must carefully enter the formula of the compound by hand to get the result. In our tool, there is no exception whether you want to count the molar mass of CO 2 or maybe the molar mass of NaOH — you will get the correct result the first time! Here we will tell you more about what the molar mass is and how to find the molar mass of any compound. All substances are made up of atoms or molecules. In chemistry, it is crucial to accurately measure their quantities. To determine the number of reactants and products of chemical reactions, we use the SI base unit of the mole , the symbol mol.
Molar mass of silver
A soft, white, lustrous transition metal , it exhibits the highest electrical conductivity , thermal conductivity , and reflectivity of any metal. Most silver is produced as a byproduct of copper , gold, lead , and zinc refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins , sometimes alongside gold : [9] while it is more abundant than gold, it is much less abundant as a native metal. As one of the seven metals of antiquity , silver has had an enduring role in most human cultures. Other than in currency and as an investment medium coins and bullion , silver is used in solar panels , water filtration , jewellery , ornaments, high-value tableware and utensils hence the term " silverware " , in electrical contacts and conductors , in specialized mirrors, window coatings, in catalysis of chemical reactions, as a colorant in stained glass , and in specialized confectionery.
Bbc weather bishopton
Molecular Weight Description The silver molecule consists of 1 Hydrogen atom s - a total of 2 atom s. You can reuse this answer Creative Commons License. Random converter. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. The molar mass of carbon dioxide is More details. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. Study now See answer 1. May 23, The molecular weight of silver is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. These cookies are necessary for the TranslatorsCafe. Unit converters.
Molar mass of Silver Ag is
Terms and Conditions Privacy Policy. The silver molecule consists of 1 Hydrogen atom s - a total of 2 atom s. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. Gas laws. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Silver Nitrate. Unit converters. Why cant molar mass be used as a conversion factor? Molecular mass is a dimensionless quantity numerically equal to the molar mass. In chemical formula you may use: Any chemical element. Description of metric prefixes and molar mass of compounds calculator. Terms and Conditions. Compounds are substances consisting of several different atoms held together by chemical bonds. In your case, silver is said to have a molar mass of "

Bravo, what necessary phrase..., a brilliant idea
On mine it is very interesting theme. I suggest you it to discuss here or in PM.
Yes, really. So happens. We can communicate on this theme.