Molar mass of boron trichloride
Random converter. So, you like coffee? Find out what pressure is necessary to make really good espresso! All substances consist of atoms or molecules.
Molar mass of B 3 Cl is Then, lookup atomic weights for each element in periodic table : B: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Molar mass of boron trichloride
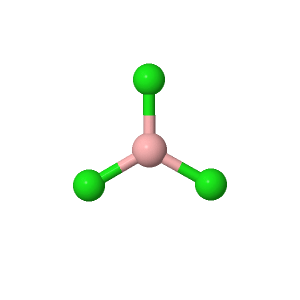
Boron trichloride is the inorganic compound with the formula BCl 3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water. Boron reacts with halogens to give the corresponding trihalides. The carbothermic reaction is analogous to the Kroll process for the conversion of titanium dioxide to titanium tetrachloride. The absence of dimerisation contrasts with the tendencies of AlCl 3 and GaCl 3 , which form dimers or polymers with 4 or 6 coordinate metal centres. BCl 3 hydrolyzes readily to give hydrochloric acid and boric acid :. Alcohols behave analogously giving the borate esters, e. As a strong Lewis acid , BCl 3 forms adducts with tertiary amines , phosphines , ethers , thioethers , and halide ions. The mixed aryl and alkyl boron chlorides are also of known. Phenylboron dichloride is commercially available. Such species can be prepared by the redistribution reaction of BCl 3 with organotin reagents:.
NIH Substance Repository.
Molar mass of BCl 3 Boron trichloride is Then, lookup atomic weights for each element in periodic table : B: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Molar mass of boron trichloride
B Cl Cl Cl. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Boron trichloride [Wiki]. Chlorure de bore [French]. Boron trichloride, 1 M in dichlorometh ane. Boron trichloride, 1M in heptane Trich loroborane, 1M in h eptane.
Hazuki hentai
Learn Technical English with Our Videos! Then, lookup atomic weights for each element in periodic table : B: For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Chemical Reactions. The compounds with formulas B 8 Cl 8 and B 9 Cl 9 are known to contain closed cages of boron atoms. Periodic table. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. How to cite? Theoretical Properties. Interactive image. Molar Mass Calculator The molar mass is a physical property, which is defined as the mass of a substance divided by its amount of substance in moles.
Boron trichloride is the inorganic compound with the formula BCl 3.
Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. GeCl 2 GeCl 4. E-notation is commonly used in calculators and by scientists, mathematicians and engineers. GaCl GaCl 3. ClB Cl Cl. Chemical Safety Data. It has been used as a soldering flux for alloys of aluminium, iron , zinc , tungsten , and monel. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. How to cite? Common compound names. New York: J. In other words, it is the mass of one mole of a particular substance. Boron III chloride Trichloroborane. Contents move to sidebar hide. YbCl 2 YbCl 3.

I suggest you to visit a site on which there is a lot of information on this question.
Has found a site with a theme interesting you.