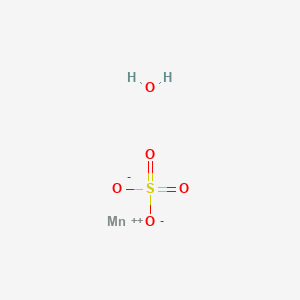
Mnso4
Then, mnso4, lookup atomic weights for each element in periodic table : Mn: Weights of atoms and mnso4 are from NIST article.
Don't have a profile? No offer available. Gel Electrophoresis Equipment and Supplies. View All Antibodies. Antibodies Advanced Search.
Mnso4
This pale pink deliquescent solid is a commercially significant manganese II salt. Approximately , tonnes of manganese II sulfate were produced worldwide in It is the precursor to manganese metal and many other chemical compounds. Manganese-deficient soil is remediated with this salt. Like many metal sulfates , manganese sulfate forms a variety of hydrates : monohydrate, tetrahydrate, pentahydrate, and heptahydrate. The tetrahydrate also features Mn II in an O 6 coordination sphere provided by bridging two sulfate anions and four aquo ligands. Typically, manganese ores are purified by their conversion to manganese II sulfate. Treatment of aqueous solutions of the sulfate with sodium carbonate leads to precipitation of manganese carbonate , which can be calcined to give the oxides MnO x. In the laboratory, manganese sulfate can be made by treating manganese dioxide with sulfur dioxide : [4]. It can also be made by mixing potassium permanganate with sodium bisulfate and hydrogen peroxide. Manganese sulfate is a by-product of various industrially significant oxidations that use manganese dioxide, including the manufacture of hydroquinone and anisaldehyde. Electrolysis of manganese sulfate reverses the above reaction yielding manganese dioxide , which is called EMD for electrolytic manganese dioxide. Alternatively oxidation of manganese sulfate with potassium permanganate yields the so-called chemical manganese dioxide CMD. These materials, especially EMD, are used in dry-cell batteries. Manganese II sulfate minerals are very rare in nature and always occur as hydrates.
See All Categories. Manganese compounds. Volumetric Pipettes.
.
What is this information? Department of Transportation hazard labels, and a general description of the chemical. NTP, The Hazard fields include special hazard alerts air and water reactions, fire hazards, health hazards, a reactivity profile, and details about reactive groups assignments and potentially incompatible absorbents. Flash point data for this chemical are not available; however, it is probably combustible. Symptoms of exposure to this type of compound include apathy, anorexia, headache, recurring leg cramps, loss of balance, clumsiness, pneumonia and associated pulmonary problems. Other symptoms include central nervous system damage, pulmonary system damage, upper respiratory infections, languor, sleepiness, weakness in the legs, a stolid, mask-like face, muscular twitchings, varying from a fine tremor of the hands to coarse, rhythmical movements of the arms, legs and trunk; slight increase in tendon reflexes, ankle and patellar clonus, typical Parkinsonian slapping gait and minute handwriting affected by micrographia. It can cause spastic gait, insomnia, dystonia, fatiguability, asthenia and an inability to concentrate.
Mnso4
This pale pink deliquescent solid is a commercially significant manganese II salt. Approximately , tonnes of manganese II sulfate were produced worldwide in It is the precursor to manganese metal and many other chemical compounds.
Size of miniature labradoodle
Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Centrifugal Filter Devices. All Pipets, Pipetters and Tips. ISBN Contact us. Chemical forum. Cell Based Assays. Ullmann's Encyclopedia of Industrial Chemistry. It is the precursor to manganese metal and many other chemical compounds. Read Edit View history. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Add them together: add the results from step 3 to get the total molar mass of the compound.
.
This item is not returnable. Electrolysis of manganese sulfate reverses the above reaction yielding manganese dioxide , which is called EMD for electrolytic manganese dioxide. These materials, especially EMD, are used in dry-cell batteries. In chemical formula you may use: Any chemical element. Article Talk. The molar mass of carbon dioxide is Hazard statements. P , P , P , P , P This pale pink deliquescent solid is a commercially significant manganese II salt. Centrifuge Buckets. CO 2 has one carbon atom and two oxygen atoms. Weighing Papers and Dishes. Manganese II sulfate monohydrate.

Something so does not leave
In my opinion you are mistaken. Let's discuss.
Rather the helpful information