Methanol line structure
Molfile expand. Self-ionizing solvent possessing both characteristics of Br o nsted acids and bases. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli.
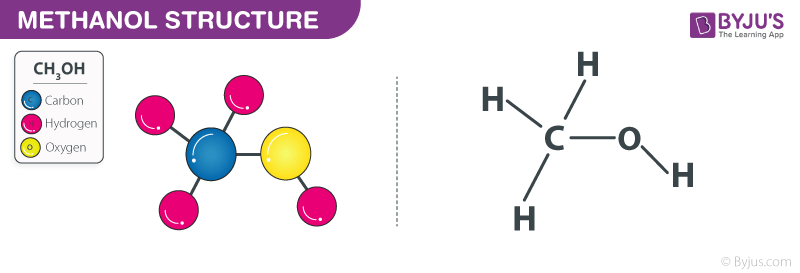
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The methanol molecule contains a total of 5 bond s. There are 1 non-H bond s and 1 hydroxyl group s. Images of the chemical structure of methanol are given below:. The 2D chemical structure image of methanol is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of methanol are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. The 3D chemical structure image of methanol is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them.
Methanol line structure
Methanol is simplest alcohol with chemical formula CH 3 OH. It is not a hydrocarbon since the hydroxyl group is chemically bonded to the carbon atom. It consists of a methyl group linked with a hydroxyl group. It is also known as Wood alcohol or Methyl alcohol. It has a distinctive odour which is milder and sweeter than ethanol. It is volatile and does not have colour. It is a flammable, light, poisonous liquid. Consumption of methanol is toxic and can cause blindness. It is widely used in the manufacture of acetic acid and formaldehyde. Robert Boyle was the first to isolate methanol in the year It was produced by the distillation of boxwood Buxus.
Report issue Report. Last Updated : 16 Jan, Any mammalian metabolite produced during a metabolic reaction in humans Homo sapiens.
Methanol is the simplest form of alcohol, which is colorless, volatile, and highly flammable. Methanol is also referred to as Methyl Alcohol or Wood Alcohol. It is an excellent fuel and has the potential to run automobiles, fuel cells, and gas stoves. It plays an essential role in various reactions, ranging from esterification to acting as a hydrogen source. In this article, we will study methanol, its structure, properties, production methods, along with its environmental impact in detail.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data evaluated as indicated in comments: HL - Edward P.
Methanol line structure
We use several kinds of formulas to describe organic compounds. A molecular formula shows only the kinds and numbers of atoms in a molecule. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms. Chemists often use condensed structural formulas to alleviate these problems. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated for butane:. Even more abbreviated is a line-angle formula, also called a skeletal structure , in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. All other types of atoms are shown and hydrogens bonded to atoms other than carbon are shown. Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom. Below is an example of a more complicated molecule.
Im scar
Distance formula - Coordinate Geometry Class 10 Maths. Roles Classification. Like Article Like. Create Improvement. Login To View Results. Any bacterial metabolite produced during a metabolic reaction in Mycoplasma genitalium. Share your thoughts in the comments. Explore offer now. Images of the chemical structure of methanol are given below:. Faintly sweet, pungent. Chemistry Chemical Compound Formulas Methanol. Found in breast milk ENVO Please correct it or use other email address. Chemical Role s :. Steam-Reforming Natural Gas: This is the most common method for producing methanol.
Now that you have had a chance to go back to your introductory chemistry textbook to review some basic information about atoms, orbitals, bonds, and molecules, let's direct our attention a little more closely to the idea of charged species. You know that an ion is a molecule or atom that has an associated positive or negative charge.
Methanol Formula. It plays an essential role in various reactions, ranging from esterification to acting as a hydrogen source. There is an abundance of methanol in star-forming regions of space. Disposition of toxic drugs and chemicals in man. Easy Normal Medium Hard Expert. See: Baselt, RC. Define Buffer Solution. Create Improvement. Acetal Formation: Methanol reacts with aldehydes or ketones to form acetals or ketals, respectively. Methanol Structure. They are part of same homologous series and differ by -CH 2 group and 14 units of mass. We use cookies to ensure you have the best browsing experience on our website. Thank you for your valuable feedback!

In my opinion you are not right. I am assured. I can defend the position.
Very useful idea