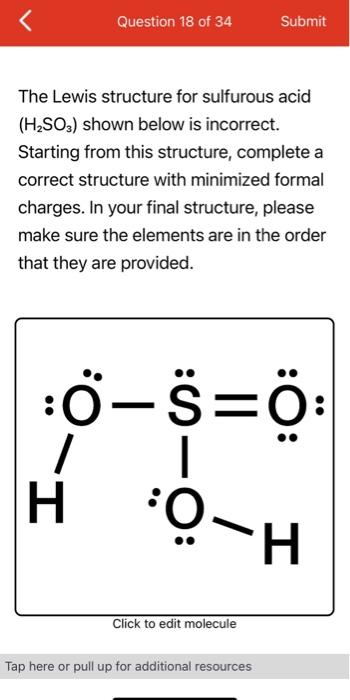
Lewis structure of h2so3
Have you heard of oxyacids of sulphur?
Submitted by Christopher J. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Choose the correct Lewis structure for the oxyacid H2SO3, called sulfurous acid.
Lewis structure of h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. There are several steps to draw the lewis structure of H 2 SO 3. Those steps are explained in detail in this tutorial. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. Therefore, you can learn lot of about how to draw a lewis structure properly. There are three elements elements in H 2 SO 3 ; hydrogen, oxygen and sulfur. Hydrogen is a group IA element and has only one electron in its last shell valence shell.
Question Solved step-by-step.
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable. Q: Write Lewis structures of simple molecules following the octet rule. A: Introduction : Lewis structures are a simple way of representing chemical structures, particularly….
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so…. Learn how to find: Hydrogen valence electrons , Sulfur valence electrons , and Oxygen valence electrons.
Lewis structure of h2so3
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom.
Latinas xnx
Introductory Chemistry For Today 8th Edition. Try Numerade free for 7 days View This Answer. The main purpose is to…. There is only one lone pair on sulfur atom in H 2 SO 3 lewis structure. Q: Draw regular Lewis structures no need to use dashed lines and wedges for each of the following…. All of the above can happen. It can even cause damage to your eyes. These are Method 1: By using sulphur dioxide. Also, there is one lone pairs on sulfur atom. A: Valence electrons of selenium and fluorine are 6 and 7 respectively. A: Sometimes, bonding in some molecules or ions cannot be described by a single lewis structure, for…. What is the total number of…. It exists in P4 tetra phosphorous form.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3.
And it neutralises sulphurous acid. Sulphurous Acid Uses There is very little to prove the existence of sulphurous acid, so there are some sulphurous acid uses. Chapter Questions Section: Chapter Questions. The structure below contains a charged carbon atom. According to octet rule, central metal atom have 8 electrons, 2. A: White phosphorous is an allotrope of phosphorous. Question Solved step-by-step. Total electron pairs are determined by dividing the number total valence electrons by two. Q: Draw the most stable Lewis's diagram for 2RO; that follows the octet rules and include the formal…. For each set of Lewis structures in the boxes below build molecular models and determine the shape of each molecule.

Willingly I accept. An interesting theme, I will take part. Together we can come to a right answer. I am assured.