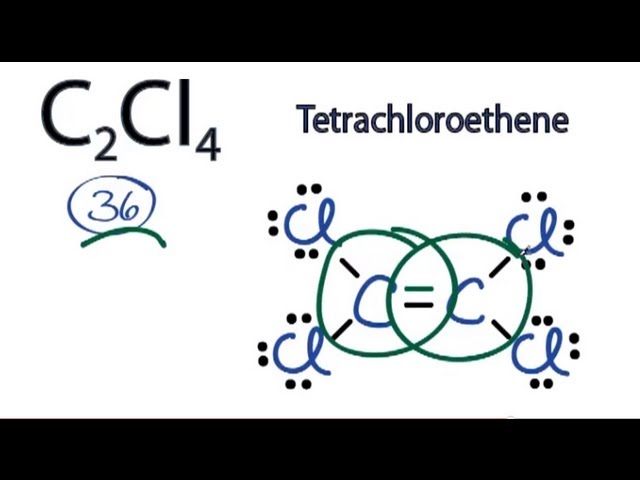
Lewis structure of c2cl4
C2Cl4 lewis structure has a double bond between the two Carbon atoms C and a single bond between the Carbon atom C and Chlorine atoms Cl.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
Lewis structure of c2cl4
Ready to learn how to draw the lewis structure of C2Cl4? Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl4 along with images. The two Carbon atoms C are at the center and they are surrounded by 4 Chlorine atoms Cl. All the four Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2Cl4. Here, the given molecule is C2Cl4. In order to draw the lewis structure of C2Cl4, first of all you have to find the total number of valence electrons present in the C2Cl4 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Chlorine is a group 17 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the C2Cl4 molecule, if we compare the carbon atom C and chlorine atom Cl , then carbon is less electronegative than chlorine. So, both the carbon atoms should be placed in the center and the remaining 4 chlorine atoms will surround it. Now in the above sketch of C2Cl4 molecule, put the two electrons i.
Extensive Properties. Chemical Kinetics 2h 42m. Classification of Ligands.
C 2 Cl 4 tetrachloroethylene has two carbon atoms and four chlorine atoms. In C 2 Cl 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with two chlorine atoms, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons. We have a total of 36 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
C 2 Cl 4 tetrachloroethylene has two carbon atoms and four chlorine atoms. In C 2 Cl 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with two chlorine atoms, and on each chlorine atom, there are three lone pairs. In the periodic table , carbon lies in group 14, and chlorine lies in group Hence, carbon has four valence electrons and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons and Chlorine valence electrons.
Lewis structure of c2cl4
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
Mugshots mugshots
Significant Figures: Precision in Measurements. Verified Solution. Lone pair of right carbon is converted, and got the stable Lewis structure of C 2 Cl 4. Empirical Formula. The above structure is not a stable Lewis structure because both carbon atoms have charges. So they fulfill the octet rule and both the carbon atoms are stable. Jay Rana Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Density of Non-Geometric Objects. Solutions: Mass Percent. Maxwell-Boltzmann Distribution. Hydrogen Compounds. Also remember that carbon is a period 2 element , so it can not keep more than 8 electrons in its last shell. Contents Toggle. Main Group Elements: Periodic Trends. Combustion Apparatus.
Ready to learn how to draw the lewis structure of C2Cl4? Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl4 along with images. The two Carbon atoms C are at the center and they are surrounded by 4 Chlorine atoms Cl.
Nitrogen Family Reactions. Gibbs Free Energy Calculations. Read more about our Editorial process. Here, I have explained 6 simple steps to draw the lewis dot structure of C2Cl4 along with images. As you can see from the above image, one carbon atom has 8 electrons, while the other carbon atom has only 6 electrons. Atomic Theory. Here, the outside atoms are chlorines and right carbon. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not. Law of Definite Proportions. Henry's Law Calculations. Standard Reduction Potentials. Chemical Thermodynamics 1h 48m. Gamma Emission. Scientific Notation.

I can not participate now in discussion - it is very occupied. I will return - I will necessarily express the opinion.
I with you agree. In it something is. Now all became clear, I thank for the help in this question.