Lewis structure k2s
Submitted by William D. We will assign your question to a Numerade educator to answer.
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Questions Courses. Do you need an answer to a question different from the above?
Lewis structure k2s
Potassium sulfide represented by the chemical formula K 2 S is a compound of potassium and sulfur that is moderately soluble in acids [1]. It is deliquescent and may spontaneously ignite in air. It is a reducing agent and an ionic compound [4]. Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS. Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9]. Potassium sulfide reacts with cobalt iii bromide to produce cobalt iii sulfide and potassium bromide [10]. Potassium sulfide reacts with dilute hydrochloric acid to produce potassium chloride and hydrogen sulfide [11]. It reacts with concentrated sulfuric acid to give potassium bisulfate, sulfur dioxide, sulfur and water [13]. The compound reacts with silver nitrate to form aqueous potassium nitrate and silver sulfide precipitate [12]. It may cause a fire hazard, so precautions must be taken not to bring it in contact with air. In the form of powder or dust, it is explosive.
Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS.
Wiki User. The S would have 6 dots its own electrons and 2 exes x which would represent the electrons given by the 2 K atoms. Calcium hydride, or CaH2, forms an orthorhombic lattice structure. For a Lewis structure of a single CaH2 molecule, simply place the Ca atom in the center single bonded to two H atoms. The formula for the compound that forms is know as barium nitrate. It has a formula of: Ba No3 2. This compound is potassium sulfide - K2S.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions. Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet two for hydrogen. If any electrons are left over, place them on the central atom. Because H atoms are almost always terminal, the arrangement within the molecule must be HOH. Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. Placing one bonding pair of electrons between the O atom and each H atom gives. Adding the remaining 4 electrons to the oxygen as two lone pairs gives the following structure:.
Lewis structure k2s
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures.
Oracion san benito contra todo mal
Time is being Van der Waals Equation. The oppositely charged ions attract each other to make CaCl 2. CaBr2 c. Was the final answer of the question wrong? In Section 4. Born-Haber Cycle. It emits toxic fumes if heated to decomposition [4]. Resources Leaderboard All Tags Unanswered. Learning Objectives State the octet rule. One page report and 7 slides needed without speaker notes but with references Question 3: Vision Statement: Indigenous Child Welfare In this task, you should imagine yourself as a worker in an agency that focusses on work with children in care A similar process occurs between Mg atoms and O atoms, except in this case two electrons are transferred:. Write a Lewis structure for each of the following ionic compounds. The need for the number of electrons lost being equal to the number of electrons gained explains why ionic compounds have the ratio of cations to anions that they do. Didn't find yours?
Potassium sulfide K2S consists of two potassium K atoms, each with 1 valence electron, and a sulfur S atom with 6 valence electrons. Sulfur achieves an octet configuration, akin to the noble gas argon, with 8 electrons. The electronegativity difference between K 0.
Inventory turnover ………………………………. Ask your question! Add To Playlist Hmmm, doesn't seem like you have any playlists. So we developed a line of study tools to help students learn their way. Ace Chat Your personal AI tutor, companion, and study partner. Paul G. About Contact. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Define ionic bond. The S would have 6 dots its own electrons and 2 exes x which would represent the electrons given by the 2 K atoms. What is the Lewis dot structure of CaH2? The compound sodium bromide is formed by the formation of ionic bonds between sodium and bromide ions.

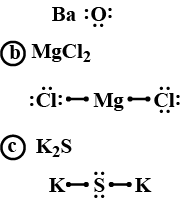
It is remarkable, very useful message
I will know, I thank for the information.
On mine the theme is rather interesting. Give with you we will communicate in PM.