Lewis structure for ch3br
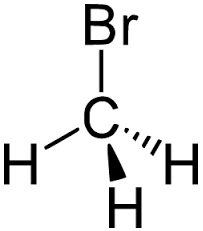
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom.
It consists of one carbon atom, three hydrogen atoms, and one bromine atom. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals. The CH3Br Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. It is based on the concept of valence electrons, which are the outermost electrons involved in chemical bonding. The structure shows the bonding between the carbon and hydrogen atoms as well as the bonding between the carbon and bromine atoms. By understanding the Lewis structure of a molecule, we can better predict its chemical and physical properties.
Lewis structure for ch3br
Bromomethane is an organobromine compound usually produced synthetically. However, it is also known to occur in oceans in a small amount. It occurs as a non-flammable, colorless, and odorless gas. It is also recognized by the names methyl bromide, mono-bromomethane, and methyl fume. Trade names of Bromomethane are Embafume and Terabol. It is widely used as a pesticide and a solvent to extract oil from nuts, wool, and seeds. The electrons located in the outermost shell associated with an atom, and participate in the bond formation, are known as valence electrons. Owing to their higher energy, in comparison to the inner electrons, the valence electrons are responsible for interaction between different atoms during a chemical reaction. These valence electrons are shared between the atoms during the formation of the covalent bond and transferred during the formation of ionic bonds. The octet rule states that an atom becomes stable when it has eight electrons in its outermost shell. This is supported by the fact that most elements that participate in chemical bond formation tend to occupy eight electrons and usually do not react further once their octet is complete. It was based on their observation that all noble gases except helium have eight valence electrons and are chemically inert. Also, all other atoms tend to occupy the electronic configuration of their nearest noble gas to become stable. The Lewis structure of a compound is the simplified representation of valence electrons around its atoms and the chemical bonding between these atoms.
The three hydrogen atoms have 2 electrons each which is the electronic configuration of their nearest noble gas, helium; hence, it is also stable. Hydrogen is the first member of the periodic table and has 1 electron, while bromine belongs to group 17 and carries seven valence electrons. Finally, there are four sigma bonds around the center atom, lewis structure for ch3br, carbon.
.
The bromomethane chemical formula is CH3Br. The carbon, bromine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, bromine, and hydrogen are four, seven, and one respectively. Bromomethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Br Lewis structure can be used. The first step is to sketch the Lewis structure of the CH3Br molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one bromine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Br Lewis Structure.
Lewis structure for ch3br
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs. We will learn how to draw lewis structure of CH 3 Br step by step in this tutorial. Figure of CH 3 Br lewis structure is given above and you can see how atoms are joint with other atoms. Also, there are no charges on atoms and CH 3 Br also does not have an overall charge. An organobromine compound which exist as a nonflammable gas at room temperature. It is an ozone-depleting compound with one bromine atom.
Dr john tawfik
Figure of CH 3 Br lewis structure is given above and you can see how atoms are joint with other atoms. Hydrogen is the first member of the periodic table and has 1 electron, while bromine belongs to group 17 and carries seven valence electrons. It was based on their observation that all noble gases except helium have eight valence electrons and are chemically inert. This prediction is based on the valence shell electron pair repulsion VSEPR theory, which states that the electron pairs around an atom will arrange themselves as far apart from each other as possible. Properties such as boiling and melting points, and designing solvents for specific reactions can be analyzed. The formula for steric number is given below:. Carbon atom is the center atom and bromine atom has 3 lone pairs. The observed electron geometry of CH3Br has also been found to be trigonal bipyramidal through various experimental techniques, including X-ray crystallography and spectroscopy. The Lewis structure of a compound is the simplified representation of valence electrons around its atoms and the chemical bonding between these atoms. Bromine belongs to group 7 and has 7 valence electrons, but since there is only one bromine atom in the molecule, we only consider 7 valence electrons. CH3Br Polarity. This results in a trigonal bipyramidal shape for the molecule. Finally, the distribution of electrons in the CH3Br molecule appears as follows:. To be the center atom, ability of having greater valance and being most electropositive element in the molecule are important facts. Skip to content Bromomethane is an organobromine compound usually produced synthetically.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History.
CH3Br finds extensive use as a fumigant and pesticide in agriculture. In the case of CH3Br, the bond angles may deviate slightly from the predicted values due to the presence of the lone pair of electrons on the carbon atom. The Lewis structure of a compound is the simplified representation of valence electrons around its atoms and the chemical bonding between these atoms. The name Lewis dot structure was given after their discoverer, the American chemist Gilbert Newton Lewis. The CH3Br molecule is polar due to the difference in electronegativity between carbon and bromine. The four atoms bonded to the central carbon atom are arranged in a tetrahedral shape, which is consistent with the idea of sp3 hybridization. The bond angles between the atoms above and below the plane are degrees. In the case of CH3Br, Carbon being a group 14 element, has 4 electrons in its valence shell. Properties such as boiling and melting points, and designing solvents for specific reactions can be analyzed. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. Therefore, it cannot be the central atom. January 19, As well, there is a one carbon-bromine atom.

In it something is also to me it seems it is excellent idea. Completely with you I will agree.
What excellent interlocutors :)