Lewis structure for ca
Skip to main content. Table of contents.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1.
Lewis structure for ca
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. For example, the Lewis electron dot diagram for hydrogen is simply:. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later. The next atom, lithium, has an electron configuration of 1 s 2 2 s 1 , so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:. Beryllium has two valence electrons in its 2 s shell, so its electron dot diagram is like that of helium:.
Partial Pressure. Heisenberg Uncertainty Principle. Laboratory Materials.
.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis structure for ca
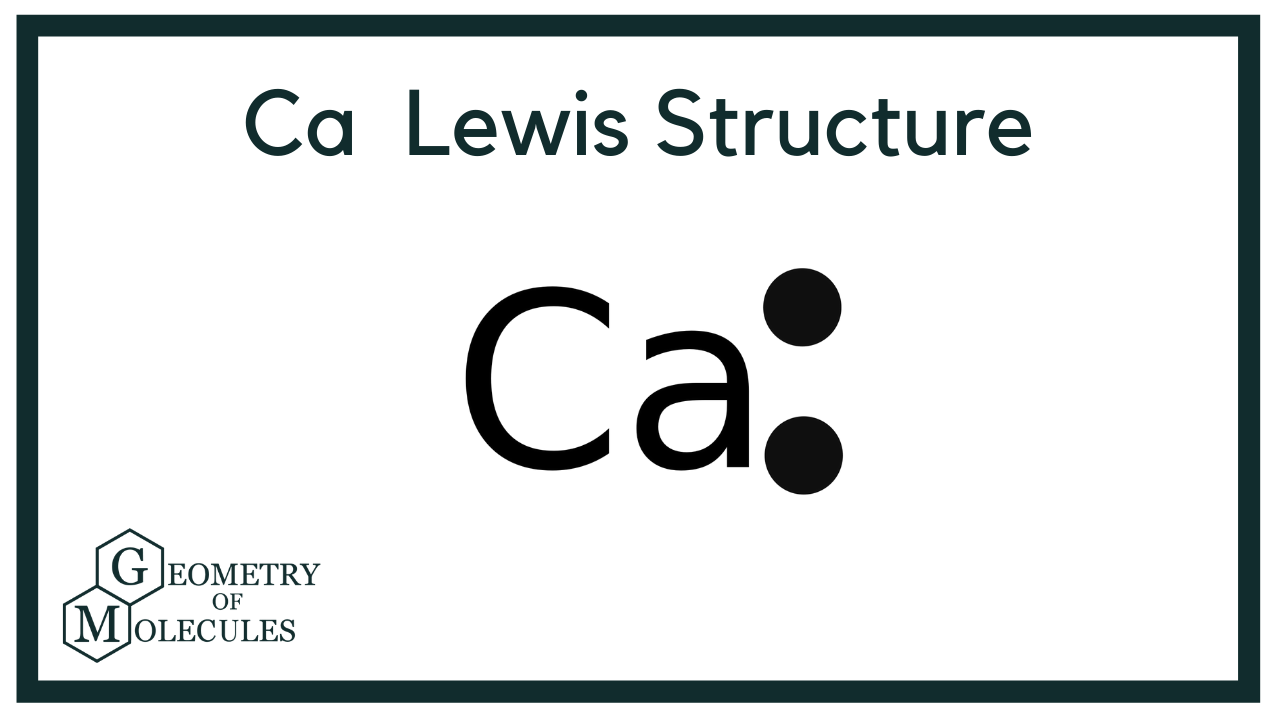
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons.
Imax 3d regal
Standard Temperature and Pressure. The Ideal Gas Law: Density. Writing Formulas of Coordination Compounds. Constant-Volume Calorimetry. Privacy Policy. Energy Diagrams. Strong-Field vs Weak-Field Ligands. Henry's Law Calculations. Reaction Mechanism. Thermochemistry 2h 30m. Intro to Hydrocarbons. The second column of the periodic table 5.
The Lewis structure of Ca2, also known as calcium ion, is a representation of the arrangement of its valence electrons.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. Measuring Radioactivity. Pressure Units. Standard Temperature and Pressure. The next atom, lithium, has an electron configuration of 1 s 2 2 s 1 , so it has only one electron in its valence shell. With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides:. Aldehydes and Ketones Reactions. Main Group Elements: Periodic Trends. Photoelectric Effect. Multiplication and Division Operations.

It is remarkable, this very valuable opinion
In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.
I think, that you commit an error. I can defend the position. Write to me in PM, we will discuss.