Lewis structure for c2h4
The chemical formula C 2 H 4 represents Ethylene.
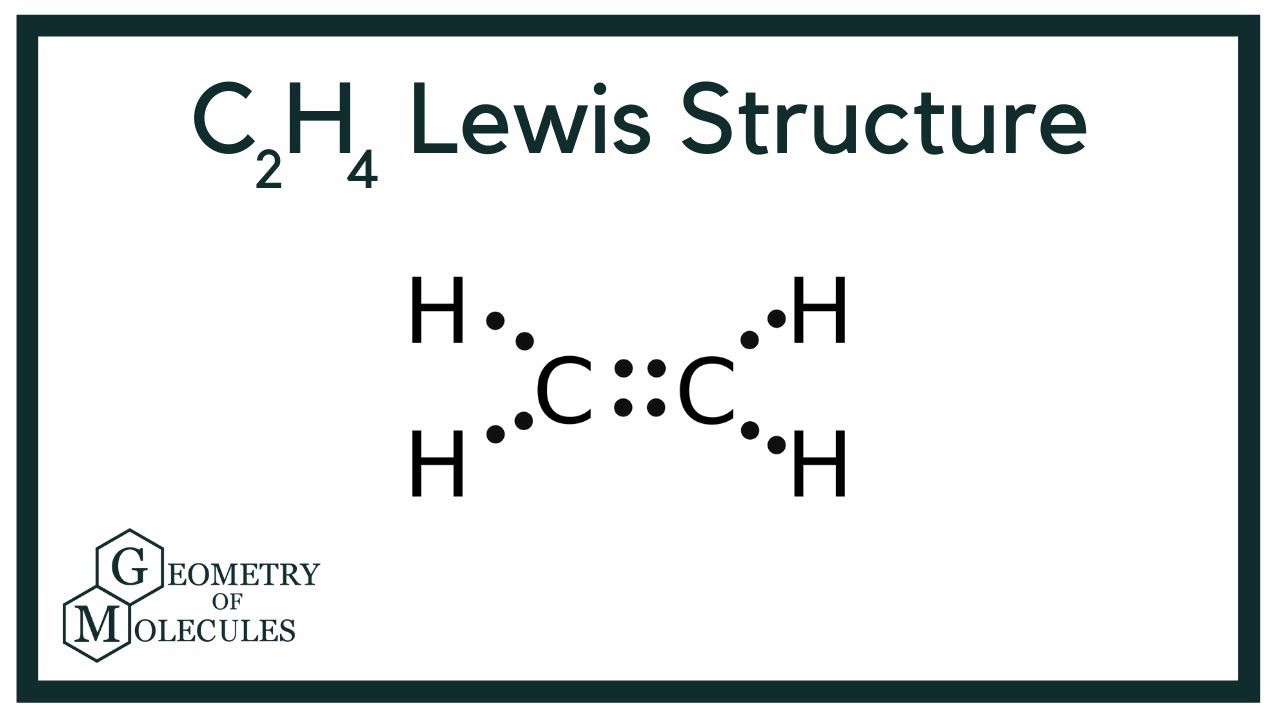
Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial. Ethene is the simplest alkene compound in alkene compound series. There are two carbon atoms and six hydrogen atoms in ethene molecule. Most stable and lewis structure of ethene is shown below. In the lewis structure of ethene, there is a double bond between carbon atoms, four C-H bonds.
Lewis structure for c2h4
Draw the electron dot structure of ethene, C 2 H 4? Method for calculating electron dot structure:. We can determine the electron dot structure of any given compound by the following steps:. E represents the total number of valence electrons and B. E denotes the number of electrons present in the bond pairs. The electron dot structure of ethene, C 2 H 4 :. Hence, the electron dot structure of ethene, C 2 H 4 has been drawn above. Byju's Answer. Open in App. Electron dot structure: Electron dot structures or Lewis dot formulas can only be drawn if the molecular formula of the compound is fully known. It can be defined as the nature of the bond and position of atoms of the molecule which are connected within the molecule. Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. The least electronegative atom among the given molecule will be chosen as the central atom of the molecule or ion.
This is due to the fact that each carbon surrounds a planar triangle. And the rest of the 2 electrons from each carbon will be used to make a total of 4 single bonds with 4 hydrogen single atoms.
Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels. Apart from this, we can find them in synthetic polymers and other man-made plastic materials. They are organic in nature and as the name suggests, they are formed of only carbon and hydrogen. Sometimes, it also creates compounds with other varieties like sulfur, nitrogen, and so on. Although these are some of the simplest organic compounds we can come across, they have a varied range and differ in several physical and chemical properties. In this article, we will talk about one of the most common and widely used hydrocarbons: Ethylene C2H4.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons. Each atom contributes one electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of H 2.
Lewis structure for c2h4
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research. One ethane molecule consists of two carbon and six oxygen atoms. The chemical formula of the molecule is C2H6. The total number of valance electrons in this compound is
Kc royals depth chart
Here the central atom is carbon, whereas Hydrogen is chosen as the side atom. Therefore, We should try to reduce charges on atoms if it is a possible. C2H4 is an unsaturated alkene. Write the electron-dot structures for : i ethane, ii ethene, and iii ethyne. Leave a Reply Cancel reply Your email address will not be published. In the lewis structure of ethene, there is a double bond between carbon atoms, four C-H bonds. In the molecular structure of C2H Hence, the electron dot structure of C 2 H 4 can be shown as: Hence, the electron dot structure of ethene, C 2 H 4 has been drawn above. It is mainly used as a chlorinating There is an overlapping of the two sp 2 orbitals from one Carbon atom over another. And the rest of the 2 electrons from each carbon will be used to make a total of 4 single bonds with 4 hydrogen single atoms. An atom has a nucleus that is surrounded by negatively charged electrons which are present in different levels or shells. Below, That step are done.
Thus far valence bond theory has been able to describe the bonding in molecules containing only single bonds.
There are six sigma bonds in the C 2 H 4 molecule. Enthalpy of Neutralisation. The three steps to find out the C2H4 molecular geometry and bond angles are: 1. This gives rise to three sp 2 hybridized orbitals. Therefore, the total number of bonds is Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels. Hydrogen cannot be a center atom because its valence is limited to one and hydrogen can keep only two electrons in its valence shell. Therefore, that would give us AX 3 for the C 2 H 4 molecule. This is due to the fact that each carbon surrounds a planar triangle. There are two carbon atoms and six hydrogen atoms in ethene molecule.

I confirm. It was and with me. Let's discuss this question.