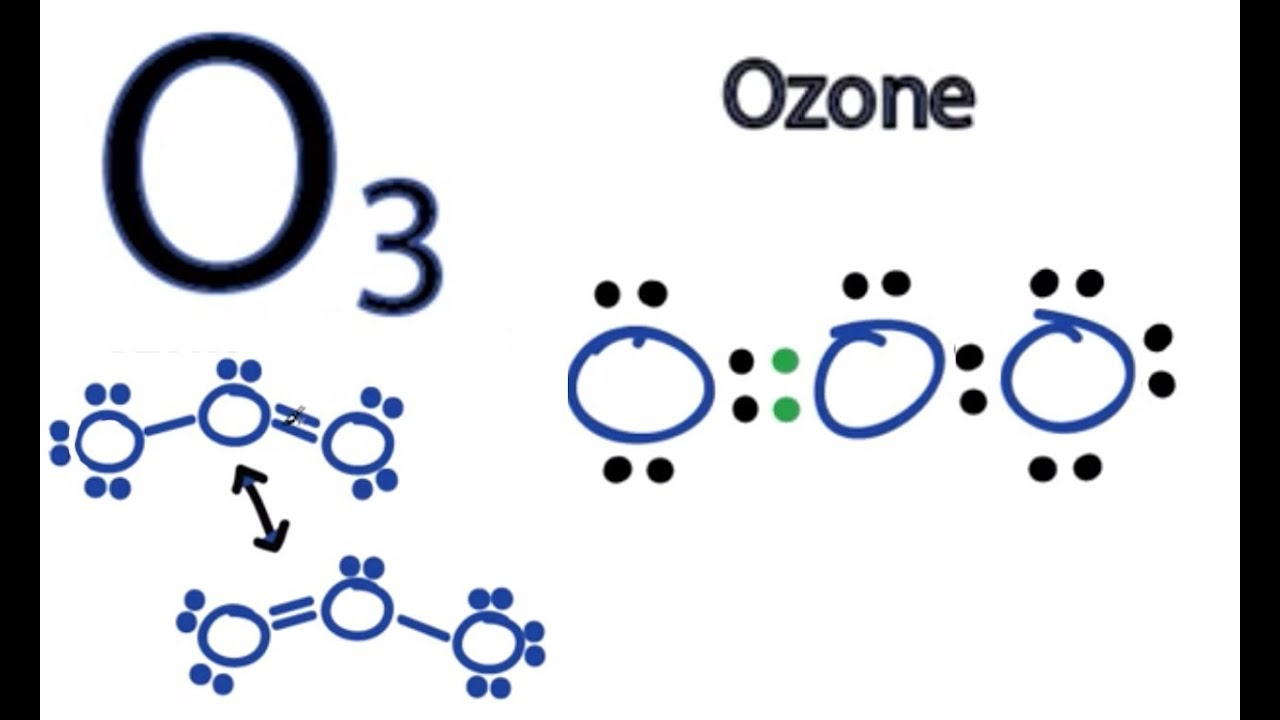
Lewis dot structure for o3
Because, around the central oxygen, lewis dot structure for o3, there are 5 electrons 2 from the double bond, 1 from the single bond, and 2 from the lone pairwe assign this centre a positive charge, and of course we can assign each terminal oxygen a negative charge alternately by resonance. What do we find experimentally? Thus, by simply knowing how to draw a Lewis structure, counting the electrons, and using VSEPRwe have predicted the structure of a gaseous molecule, which we can't see, but we can smell. I think that is pretty clever given the short!
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs. In short, these are the steps you need to follow for drawing a Lewis structure :.
Lewis dot structure for o3
This article in whole includes the details on the topic and a short note on the resonance structure of O3. This article also includes the topics like bond length and major and minor contributors of resonance. Resonance structures are a more accurate representation of a Lewis dot structure than Lewis dot structures because they clearly illustrate the bonding between molecules. Not all resonance structures are created equal; some are superior to others. The better ones have the fewest formal charges, the most electronegative atoms have the most formal charges, and the structure maximizes bonding. The more resonance forms a molecule has, the more stable the molecule is. They are connected by a double-headed arrow, indicating that the true structure is between the resonance structures. Curved arrow notation was employed to depict the flow of electrons from one resonance type to the next. Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Ozone has one double bond and one single bond in its Lewis structure. Additionally, two oxygen atoms in the O 3 Lewis structure have charges. The Lewis structure of O 3 can be deduced in various phases starting with the valence electrons of oxygen atoms. This lesson walks you through each step of drawing the Lewis structure of O 3.
Negative charge, if any, should be applied to the most electronegative atoms, and positive charge, if any, should be applied to the most electropositive atoms. Chemical Bonding O3 Lewis Structure. Related articles.
.
Discover the basics of O3 ozone with our easy-to-understand guide. Ideal for those exploring chemistry concepts or environmental studies. Lewis structures are a way to represent the bonding and electron distribution in a molecule. In this blog post, we will go through the step-by-step process of drawing the Lewis structure for O3, also known as ozone. Ozone, with the chemical formula O3, is a molecule that consists of three oxygen atoms. Drawing the Lewis structure of O3 helps us understand its bonding and determine its shape and properties. To start drawing the Lewis structure for O3, we need to determine the total number of valence electrons in the molecule. Valence electrons are the electrons in the outermost shell of an atom. In the case of O3, each oxygen atom contributes 6 valence electrons since oxygen is in Group 16 of the periodic table. In O3, one oxygen atom will be the central atom, while the other two oxygen atoms will be bonded to it.
Lewis dot structure for o3
Due to vast global warming and the rapid increase of temperature on earth, the ozone layer of the stratosphere has a hole in it. This causes severe climate change and environmental damage. A pale blue gas with a molar mass of Formed from the dioxide molecule, this molecule having three oxygen atoms is very crucial from the chemistry point of view. If you want to dive into his molecule, let us fasten our seat belts! Because I am going to make you travel through all the essential concepts and explanations related to bonding within ozone. To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure:. The initial step towards forming this structure is to find out the total number of valence electrons.
Chair anal
As a result, the triple bond formed by the three oxygen atoms will exhibit strong single bond properties. Next, check if the atoms have octet. The middle oxygen is surrounded by six electrons, and we move one of the lone pairs to make a double bond: And now all three oxygens have complete octet: Next, we check for formal charges. Molecules containing three electron pairs have a trigonal planar domain shape. Negative charge, if any, should be applied to the most electronegative atoms, and positive charge, if any, should be applied to the most electropositive atoms;. Keep in mind that the actual structure of the molecule is intermediate between the two or more resonance structures and is called a resonance hybrid. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application. They are connected by a double-headed arrow, indicating that the true structure is between the resonance structures. If any atoms lack an octet, make a double or triple bond to give them an octet. Additionally, two oxygen atoms in the O 3 Lewis structure have charges. What is zone refining and what is its significance in manufacturing transistors? The ozone O 3 molecule is composed of a core oxygen atom that is linked singly to one oxygen atom and doubly to another. Notice also that the molecular geometry of ozone is bent because the central atom is connected to two atoms and has one lone pair. With rules outlined in rough order of decreasing importance, substantial contributors are often structures that adhere to the octet rule to the greatest extent feasible 8 valence electrons around each atom rather than deficits or surplus, or 2 electrons for Period 1 elements ; have the greatest possible amount of covalent bonds; carry the fewest number of formally charged atoms possible, with the spacing of opposite and like charges minimized and maximized, respectively; Negative charge, if any, should be applied to the most electronegative atoms, and positive charge, if any, should be applied to the most electropositive atoms; do not significantly differ from idealized bond lengths and angles for example, the relative insignificance of Dewar-type resonance contributors to benzene ; Locally, preserve aromatic substructures while avoiding anti-aromatic ones.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements.
With rules outlined in rough order of decreasing importance, substantial contributors are often structures that adhere to. Ozone has one double bond and one single bond in its Lewis structure. After drawing the Lewis structure of NH 3 , the shape of the O 3 molecule can be determined. We now have 14 valence electrons left, and since the middle oxygen has two bonds while the terminal oxygens have one, we add them to terminal oxygens as two pairs:. Because it's a simple predictor of molecular shape. Notify me of followup comments via e-mail. Not all resonance structures are created equal; some are superior to others. When various approaches of constructing a Lewis dot diagram that satisfies the octet rule exist, resonance structures arise. As a result, the triple bond formed by the three oxygen atoms will exhibit strong single bond properties. Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Thus, by simply knowing how to draw a Lewis structure, counting the electrons, and using VSEPR , we have predicted the structure of a gaseous molecule, which we can't see, but we can smell. Sum the valence electrons from all the atoms.

Bravo, brilliant idea