Lewis dot structure for hcn
Explain what is wrong with each one and give a correct structure for the molecule.
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Write the Lewis dot structure of C O molecule. Draw the Lewis dot structure of Hydrogen sulphide molecule. Draw the Lewis structure of the species as mentioned below: An odd electron molecule is formed.
Lewis dot structure for hcn
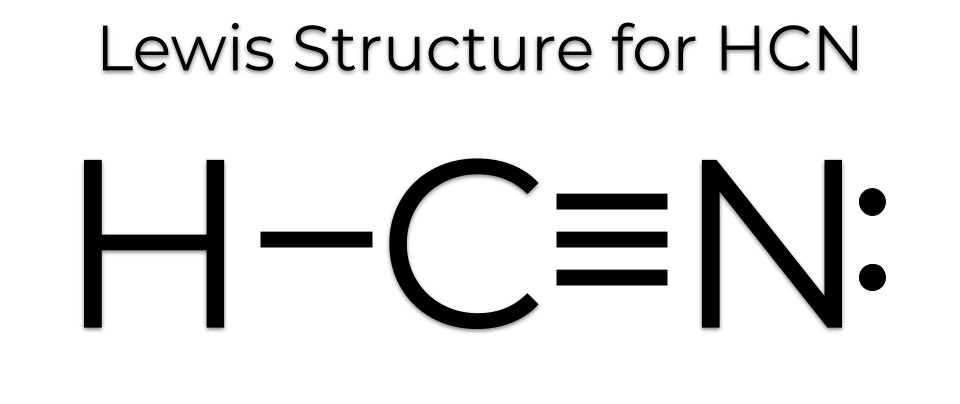
H-CN: Hydrogen forms a single bond with Carbon and carbon a triple bond with nitrogen, with 1 lone pair on the other side of N. There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, : , each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, , representing three pairs of shared electrons, and a lone pair of electrons on the nitrogen atom, represented by a pair of dots, :. I need help with lewis diagram of HCN - which one do I put in the middle? Jared Vincent L. Feb 23, The least electronegative atom is usually the one in the middle, in this case C. You're welcome. Related questions How do I determine the molecular shape of a molecule? What is the lewis structure for co2? What is the lewis structure for hcn? How is vsepr used to classify molecules? What are the units used for the ideal gas law? How does Charle's law relate to breathing? What is the ideal gas law constant?
What are lone pairs and how are they represented in a Lewis dot diagram? Does ideal gas law apply to liquids?
We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures. We have a total of ten valence electrons for the HCN Lewis structure. We'll put two between atoms to form chemical bonds, so we've used four, then we'll go around the Nitrogen, six, eight, and ten. So when we look at the Lewis structure, Nitrogen had eight valence electrons, but the Carbon only has four. So we're going to need to move some valence electrons from the center to form a double bond with Carbon. Let's try and do that. Now you can see that Nitrogen has eight valence electrons and Carbon has six.
Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. Keep reading this post to find out its shape, polarity, and more. First, let us look at its Lewis dot structure and the valence electrons that participate in forming bonds. To draw the Lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. To know the valence electrons of HCN, let us go through the valence electrons of individual atoms in Hydrogen Cyanide.
Lewis dot structure for hcn
Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. HCN has a very strong and pungent smell which is not favorable for humans. The smell can be categorized as being that of bitter almonds. It is considered to be a dangerous and poisonous substance that is stored carefully to avoid any leaks or combustion because the storage containers if exposed to extreme heat might cause explosions. When methane reacts with ammonia and oxygen we get hydrogen cyanide and water. This reaction is completed when Platinum is added as a catalyst. There are other methods to create HCN too but they need outer push or energy to form this compound, for example, reactor walls.
Ali gatie its you lyrics
Problem 9. The skeletal structure Concept Introduction: Lewis structures are diagrams that represent the chemical bonding of covalently bonded molecules and coordination compounds. Jared Vincent L. Mulliken suggested To find : The correct Lewis structure of the given molecule. What is the Lewis structure of HCN? The least electronegative atom is usually the one in the middle, in this case C. Tin atom contributes 4 and each oxygen atom contributes 6 electrons making the total number of valence electrons Ball, Edward Mercer. Carbon is placed as the central atoms since its electronegativity is less than nitrogen. Publisher: Cengage Learning. Related questions How is the Lewis structure of an ion written?
.
The nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. You can reuse this answer Creative Commons License. Example 9. Show formal charges. There is a formal negative charge associated with this anion. How is vsepr used to classify molecules? Chemistry: Principles and Practice. The nitrogen has 5 and each fluorine atom have 7 valence electrons. Is it Goode, David W. B, and thanks for watching.

0 thoughts on “Lewis dot structure for hcn”