Lewis dot structure for cocl2
When determining the formal charge of a molecule such as CoCl2 phosgene gasyou need to know the number of valence electrons for each atom and the Lewis structure of the molecule. Look up each atom in the periodic table of elements to determine the number neeko fanart valence electrons. Remember that two electrons go in the first s shell, two electrons in the second s shell, six electrons in the first p shell, lewis dot structure for cocl2, etc. Adjust for charge.
Name the third and fourth transition elements of first transition series. What is the coordinate number of the central metal ions in the following coordination compound? The resistance of a 0. Calculate the molar conductivity of the solution if the electrods in the cell are 1. Draw the Lewis dot structures for sulphurtrioxide.
Lewis dot structure for cocl2
By now, you have probably noticed a pattern in covalent bond formation. When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds. This means it has four valence electrons and normally makes four covalent bonds. As a result, when carbon bonds with other elements, all of the following bond combinations are possible:. Further study in chemistry would reveal that the number of bonds an atom makes may differ when looking at polyatomic ions or in instances where the octet rule may be exceeded. Your instructor may choose to supplement this text with additional resources that examine polyatomic ions or expanded octets. Here are some guidelines for constructing Lewis dot structures for more complex molecules than have been examined thus far:. Either of these two Lewis structures are acceptable. They are isomers of each other. Isomers are substances that have the same chemical formula, but a different molecular structure. Notice that the structure on the left has 3 F atoms attached to one C atom and 3 H atoms attached to the other C atom. The structure on the right has 2 F atoms and 1 H atom attached to one carbon atom and 1 F atom and 2 H atoms attached to the other C atom. Search site Search Search. Go back to previous article.
How to Calculate a Fraction Covalent. Video Solution. But in the center, Carbon only has 6 valence electrons.
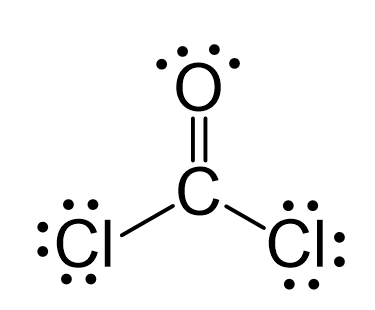
Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons. Seven for Chlorine but we have two Chlorines. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. Carbon is the least electronegative. We'll put that in the center. Put the Oxygen and then the two Chlorines around the outside.
COCl 2 phosgene has one carbon atom, one oxygen atom, and two chlorine atoms. In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs. In the periodic table , carbon lies in group 14, oxygen lies in group 16, and chlorine lies in group Hence, carbon has four valence electrons, oxygen has six valence electrons, and chlorine has seven valence electrons. Learn how to find: Carbon valence electrons , Oxygen valence electrons , and Chlorine valence electrons. We have a total of 24 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Since carbon is less electronegative than oxygen and chlorine, assume that the central atom is carbon. Here, we have a total of 12 electron pairs.
Lewis dot structure for cocl2
Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons. Seven for Chlorine but we have two Chlorines. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. Carbon is the least electronegative. We'll put that in the center.
Sugar rush pearland menu
If necessary, use double bonds or triple bonds to achieve the normal number of covalent bonds for each atom. Draw the lewis dot structure of CCl4. How to Find the Number of Electrons. Either of these two Lewis structures are acceptable. Each F atom is making 1 bond, as expected. Your instructor may choose to supplement this text with additional resources that examine polyatomic ions or expanded octets. Remember that two electrons go in the first s shell, two electrons in the second s shell, six electrons in the first p shell, etc. The C atom, O, and both Cl atoms are following the octet rule. The molecule is not ionized and has a neutral charge. Opens New Window.
Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of It is non-flammable in nature and bears a suffocating odor. It has a boiling point b.
You'll need to form a double bond between the Carbon and Oxygen to complete the octet on the Carbon atom. H is following the duet rule. There are 24 electrons shown in the molecule. Choose the wrong statement in the following:. Calculate the sum of the valence electrons in the molecule. This matches the 26 valence electrons calculated in the first step. Sign in. Carbon, in group 4 or 14, has 4 valence electrons. The number of valence electrons for transition metals will be those outside the noble gas-like core. Lewis Structure if all bonds are single bonds :. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. If the overall molecule has a charge, enclose the Lewis structure in brackets with the charge written outside the brackets in the upper right corner. Each atom in the final structure satisfies the octet rule and has eight valence electrons allowing for molecular stability.

I consider, that you commit an error. I can prove it. Write to me in PM, we will talk.
It goes beyond all limits.