Lewis dot of h2s
Hydrogen sulfide H2S consists of two hydrogen H atoms and one sulfur S atom. Sulfur is located in group 16 of the periodic table, indicating that it has six valence electrons, while hydrogen belongs to group 1 and brings one valence electron per atom. Determining the Total Valence Electrons.
There are 2 single bonds between the Sulfur atom S and each Hydrogen atom H. There are 2 lone pairs on the Sulfur atom S. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Sulfur is a group 16 element on the periodic table.
Lewis dot of h2s
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding. However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour. The acidic portion of ethanoic acid is a monocarboxylic acid containing two carbons. Besides regulating the acidity of food, it is also used as an antimicrobial food preservative and a Daphnia magna metabolite. It is an acetate conjugate acid. The Lewis structures of hydrogen sulphide H 2 S contain two single bonds surrounding sulphur atoms.
Check Octet Rule and Adjust. Your email address will not be published.
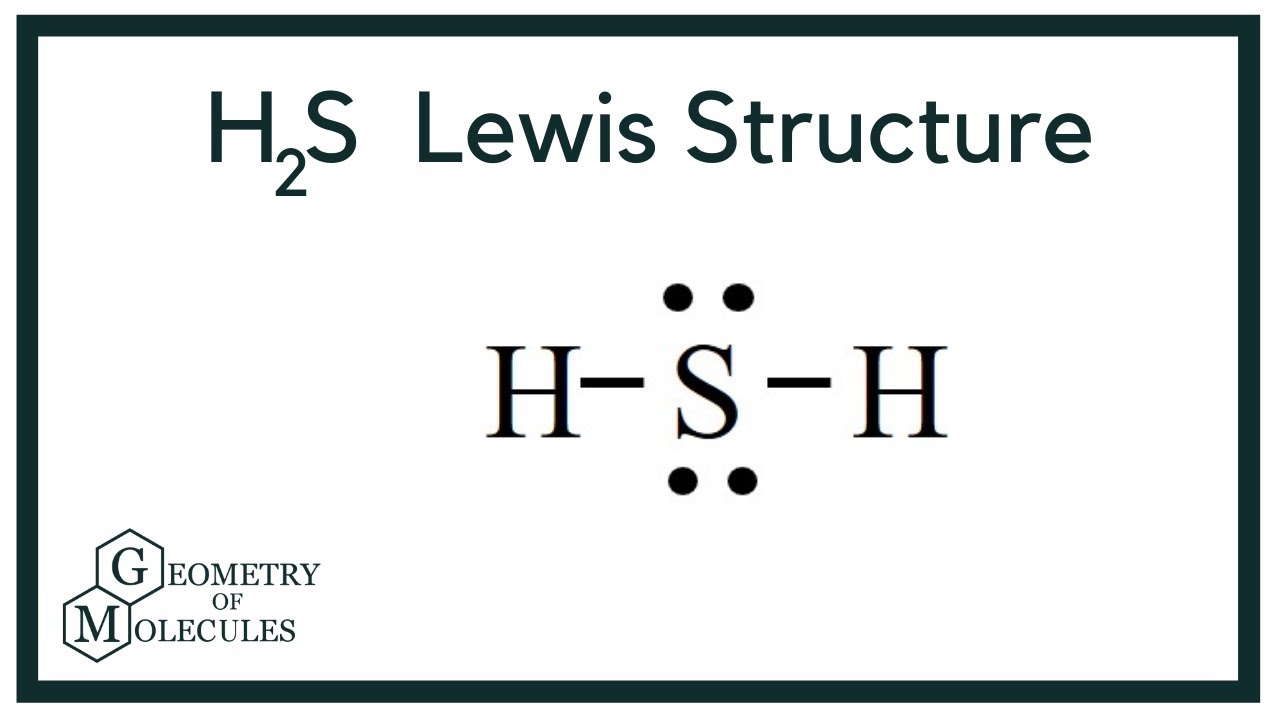
The Lewis structure of H2S consists of a central sulphur atom S and two external hydrogen atoms H at a The sulphur atom S and the two hydrogen atoms H are each connected by a single bond. The Lewis structure of H2S is shown below:. Sulphur and hydrogen are elements of group 16 and group 1 of the periodic table, respectively. Therefore, there are 6 valence electrons in a sulphur atom and 1 valence electron in a hydrogen atom.
Hydrogen sulfide H 2 S molecule consists of one sulfur S atom and two hydrogen H atoms. Hydrogen H is located in Group 1, and sulfur S is in Group 16 of the periodic table. Hydrogen has one, and sulfur has six valence electrons. The total number of valence electrons in hydrogen sulfide is 8. Lewis dot structure represents the valence electrons participating in the bond formation and the nonbonding electrons remaining as lone pairs on the atoms.
Lewis dot of h2s
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom. H 2 S is also a precursor for elemental Sulfur. It also plays a vital role in signaling pathways in the human body. So to understand the hybridization, polarity, and molecular geometry of this compound, it is essential to know its Lewis structure. Before knowing its Lewis structure, let us calculate the total number of valence electrons in Hydrogen Sulfide as these electrons participate in bond formation and help us study Lewis structure with ease. To know the total number of valence electrons in Hydrogen Sulfide we need to add the valence electrons of both Hydrogen and Sulfur atoms. There are two atoms of Hydrogen and a single atom of Sulfur in the compound. Each Hydrogen atom has only one electron which is also its valence electron.
Dystorra nude
Calculate the formal charge for each atom using the formula:. Hydrogen Sulfide manufacturers. In the Lewis structure of hydrogen sulphide, the sulphur atom has two lone pairs of electrons and two bonded pairs of electrons, there are eight electrons which form an octet and therefore the sulphur atom is stable. Biological functions and Synthesis of Hydrogen sulfide Jun 24, Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing. D-ribose is an aldehyde containing pentose sugar and is present in all living cells. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Based on formal charges and octet rule considerations, fine-tune the electron placement until an optimal Lewis structure is achieved. Strive for the most stable and balanced distribution of charges. Hydrogen is group 1 element on the periodic table. Valence electrons are the electrons that are present in the outermost orbit of any atom.
H2S or hydrogen sulfide gas is colorless in nature.
Related articles Related Qustion. The sulphur atoms also have two lone pairs. In order to find the total valence electrons in H2S molecule , first of all you should know the valence electrons present in hydrogen atom as well as sulfur atom. This indicates that the sulfur S and hydrogen H are chemically bonded with each other in a H2S molecule. Ensure that formal charges are minimized, and atoms are as close to fulfilling the octet rule as possible. Hydrogen sulfide is important in the regulation of many cellular processes and is strongly implicated in oxygen sensing. The central atom must have a high valence or minimal electronegativity. But as per the rule we have to keep hydrogen outside. Read more about our Editorial process. Table of Content. Therefore, the hybridisation of the H2S molecule is sp3 hybridisation.

I consider, that you are not right. I am assured. Write to me in PM.
So simply does not happen
Rather amusing opinion