Lewis diagram for hcooh
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it, lewis diagram for hcooh. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0.
Lewis Dot Structures. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of HCN. Is the octet roule obeyed in these structures? Write the Lewis dot structure for the following covalent molecules: S i H 4.
Lewis diagram for hcooh
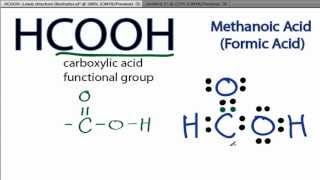
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 18 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Now we have to choose the central atom from carbon and oxygen. Place the least electronegative atom at the center. Since carbon is less electronegative than oxygen, assume that the central atom is carbon. Here, we have a total of 9 electron pairs. And four bonds are already marked.
Q: Consider the following scheme. About author.
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Carbon is group 14 element on the periodic table.
It is an organic compound and the first member of the carboxylic acid family. Formic acid was isolated from the distillation of ant bodies in earlier times and it is also produced from methanol industrially. The molar mass of formic acid is Formic acid is a colorless liquid with a pungent and penetrating odor. It is highly soluble in water and polar solvents. It exists as a hydrogen-bonded dimer in the vapor phase as well as in hydrocarbons.
Lewis diagram for hcooh
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats.
Biryani factory menu
Always start to mark the lone pairs from outside atoms. More Than Just We take learning seriously. No Try it. Crystals b. A: Identify the product of the given conditions First of all, we have an H in front, and that means it's going to be an acid. Pr forms a complex with two ammonia molecules, one SCN and a Cl ion. Welcome Back. Signup to see your scores go up within 7 days! So we have to only mark the remaining five electron pairs as lone pairs on the sketch. If we compare the electronegativity values of carbon C and oxygen O then the carbon atom is less electronegative.
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom.
Hydrogen is group 1 element on the periodic table. A: Identify the product of the given conditions Chapter Questions Section: Chapter Questions. Reviewed By Expert Numerade Educators. Learn more about Theories of Bonding. Edu cation Rev olution. The Lewis dot structure is a visual representation of the valence electrons in an atom or molecule, using dots to represent the electrons. Give one example each for a compound with a an ionic bond b a cov The Best you need at One Place. Try it in the Numerade app? View all answers. Q: Which symbols would complete this nuclear equation? Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. In this case, we need to form a double bond between C and one of the O atoms.

0 thoughts on “Lewis diagram for hcooh”