Lewis diagram for c2h6
Since C has 4 valence electronsand each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule.
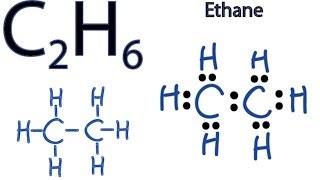
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons.
Lewis diagram for c2h6
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside. Thus, the carbon atom C is the central atom and the hydrogen atom H is the outer atom. For the C2H6 molecule, the total number of pairs of electrons is seven. In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. In addition, we must check that the central carbon atom C is stable, and we can see from the above steps that both carbon atoms are forming an octet. This means that they have 8 electrons. Therefore the central carbon atom is also stable.
Therefore, this structure is the stable Lewis structure of C 2 H 6.
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Ethane is an organic compound with a chemical formula of C2H6. It is a colorless and odorless molecule that exists as a gas at the standard room temperature. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has quite many uses in various industries and has one of the most simple hydrocarbon structures. It is also referred to as methyl methane, Bimethyl, and Dimethyl. Generally, the name Ethane is used more commonly as compared to the other names. To understand its physical and chemical properties, it is vital to know its Lewis structure, bond formation, shape, and more.
Lewis diagram for c2h6
C2H6, known as ethane, is a saturated open-chain hydrocarbon or we can say that it comes under the alkane family. Hydrocarbon is an organic compound, which contains only carbon and hydrogen. Saturated hydrocarbons are those hydrocarbons, which contain carbon-hydrogen and carbon-carbon single bonds. Saturated hydrocarbons are further classified into alkane open chain of carbon atoms and cycloalkane closed chain of carbon atoms.
How long is disney on ice 100 years of wonder
He has a good conceptual knowledge on different educational topics and he provides the same on this website. In order to check the stability of the central carbon C atoms, we have to check whether they are forming an octet or not. You can reuse this answer Creative Commons License. Impact of this question views around the world. So, the two C atoms are placed in the center of the molecule. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. The above steps have reached the stability of the C2H6 Lewis structure without further changes. Also, in step 1 we have calculated the total number of valence electrons present in the C2H6 molecule. C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Next: COCl 2 Lewis structure. The uses and side effect of Diclofenac sodium.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be.
You can see the number of bonding electrons and nonbonding electrons for each atom of C2H6 molecule in the image given below. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. This means that they have 8 electrons. Step 4 Stability of structure In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. Step 2 Identify the central atom The central atom must satisfy the principle of less electronegativity. What are some examples of Lewis structures? According to this theory, the shape and geometry of the molecule depend on the number of bonding electrons and lone pair of electrons. Jay Rana. What is an example of a Lewis structures practice problem? In short, now you have to find the formal charge on carbon C atoms as well as hydrogen H atoms present in the C2H6 molecule. These outer hydrogen atoms are forming a duplet and hence they are stable. Thus, C2H6 is sp3 hybridized.

On your place I would go another by.
Between us speaking, in my opinion, it is obvious. Try to look for the answer to your question in google.com
I consider, that you commit an error. I suggest it to discuss.