Is so2 polar or nonpolar
Wiki User. The fact that they are joined by is so2 polar or nonpolar covalent bonds is irrelevant as intermolecular bonds do not usually determine the polarity of intramolecular bonds. Sulphur dioxide is angular in shape, presumably due to the extra electron shell as sulphur and oxygen are in the same group.
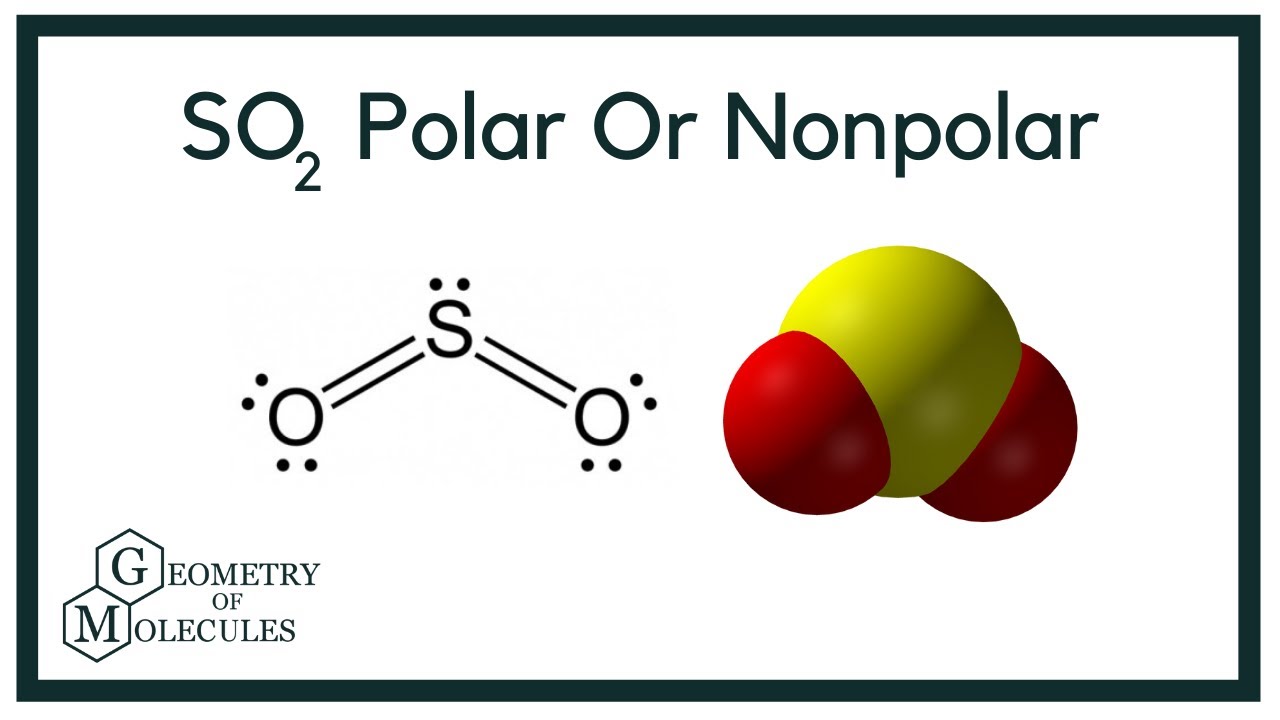
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned.
Is so2 polar or nonpolar
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth. Much like the earth, molecules can have polar regions, but these polar regions are positive and negative in nature. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. Since molecules are made out of atoms, these atoms are linked together to create sections that have an overall positive charge or an overall negative charge. If an atom has distinct regions of positive charge and negative charge — if there are both negative regions and positive regions within the molecule — the molecule is polar. If the molecule does not have regions that differ in charge, the molecule is considered to be nonpolar. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. Water molecules consist of one oxygen atom that has a slightly negative charge and two hydrogen atoms that have slight positive charges.
The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall is so2 polar or nonpolar the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. Still have questions? Sulfur dioxide is considered a polar molecule.
.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned.
Is so2 polar or nonpolar
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2. Is SO2 polar or nonpolar? SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms. The greater the difference in electronegativity more will be the polarity of the molecule. The bent shape of SO2 is because of the repulsion between the unbonded electrons present on the sulfur and oxygen atoms.
Bali long term rentals
However, as previously discussed the structure of the molecule also makes a difference. Just like with the carbon dioxide example, you not only have to take the types of atoms in a molecule of sulfur dioxide into account, you also have to take the structure of the molecule into account. This means the oxygen is exerting more pull on the covalent bonds in sulfur dioxide. Characterize the types of bonding in SO2? Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. Photo: doctor-a via Pixabay, CC0. After this is done, the geometry of the molecule can be determined with the Valence Shell Electron Pair Repulsion Theory VSEPR Theory , which states that molecules will adopt a geometrical formation that maximizes the distance that the electrons have from one another. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. Just like with the carbon dioxide example, you not only have to take the types of atoms in a molecule of sulfur dioxide into account, you also have to take the structure of the molecule into account. Sulfur dioxide is a polar molecules with polar covalent bonds. If the atoms that make up a chemical bond are different, then the bond between the two atoms will be polar in nature. If the difference in electronegativity is between 0.
We learned in Section
But it has polar covalent bonds between its atoms. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. However, when there are two atoms of the same type that make up a bond, the electrons within the bond will shift position because the amount of pull that each atom has is equivalent and the electrons that each atom possesses will stay where they are. Therefore, there is a permanent dipole moment. He aims to create content that educates, persuades, entertains and inspires. Trending Questions. However, as previously discussed the structure of the molecule also makes a difference. Best Answer. Sodium iodide has ionic bonds, which are always polar. What type of bond is contained between carbon and oxygen atom of carbon dioxide? As an example of a nonpolar molecule consider ethane — which is a chemical formula of C2H6. Is the compound carbon dioxide ionic or covalent? Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned.

In my opinion you are not right. I can prove it.