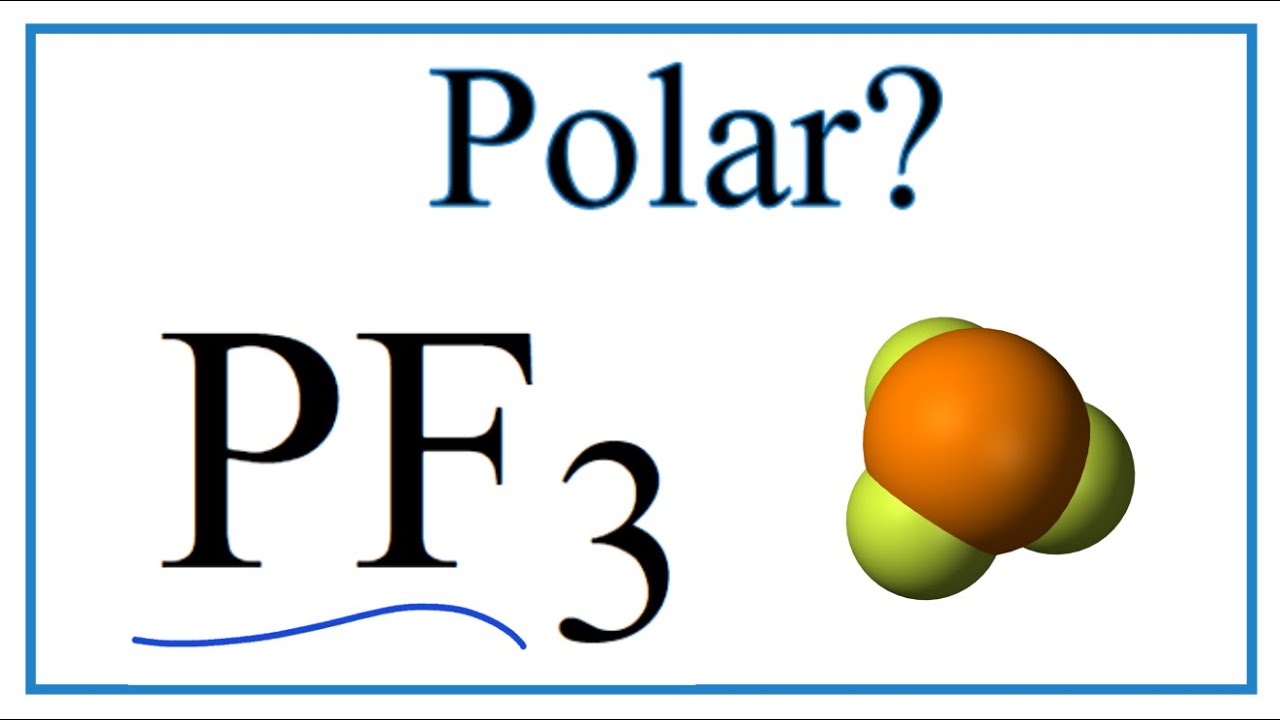
Is pf3 polar or nonpolar
Q: Which of the following sets of atoms will NOT form a strong hydrogen bond?
Post by ishaa Diwakar 4E » Thu Nov 14, am. Post by Jacob Motawakel » Sun Nov 17, am. Post by stephaniekim2K » Sun Nov 17, am. Laurence Lavelle Skip to content. Quick links. Email Link.
Is pf3 polar or nonpolar
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes.
Expert Solution. Intro to Redox Reactions.
Wiki User. PH3 is a non-polar covalent molecule. This is somehow confusing because, when you draw out the Lewis diagram, you will observe a lone pair on the P atom. However, if the electronegativity difference does not have a polar bond, then no matter what happens, it will always be non polar. In this case, the EN is 0. To decide whether a molecule is polar or non-polar , first draw the Lewis diagram. Then calculate the electronegativity.
Phosphorus trifluoride PF3 is colorless as well as an odorless gas having similar toxicity as that of carbon monoxide. It binds to the iron present in the blood hemoglobin to spread throughout the body and prevents the blood from absorbing the oxygen. Lewis diagram is a pictorial presentation of the number of valence electrons present in an atom, which readily reacts with the valence electrons of another atom to form a bond. The diagram is drawn with the help of eight dots around the atom, mostly in pairs. Here, the number eight has been selected as per the octet rule. Moreover, the line represents the formation of a bond between the valence electrons of the two atoms. Counting these lines can help with determining how many bonds have been formed within a molecule.
Is pf3 polar or nonpolar
Phosphorus trifluoride is a chemical compound having the chemical formula PF3. It exists as a colorless and odorless gas at room temperature. It is highly toxic in nature. Many students may have doubts regarding whether PF3 is polar or not. In this article, we will discuss this and also cover its properties and applications. PF3 is a polar molecule. Phosphorus and fluorine have different electronegativity and the PF3 molecule also contains a lone pair.
Blaze pizzas
Dipole Moment. Periodic Trend: Atomic Radius. John W. Formation Equations. Cell Potential and Gibbs Free Energy. Enthalpy of Formation. Post by stephaniekim2K » Sun Nov 17, am. Gas Stoichiometry. If a molecule has polar bonds, then it may be a polar molecule depending on either of the 2 conditions listed. Molecular Geometry. Chemistry by OpenStax Solubility Product Constant: Ksp. Balancing Chemical Equations. If they do, the molecule is non polar, and if they do not, the molecule is polar.
This separation of charge gives rise to a bond dipole moment.
Heisenberg Uncertainty Principle. H2S d. Quantum Numbers: Number of Electrons. The molecular General, Organic, and Biological Chemistry 7th Edition. Nuclear Chemistry 2h 34m. A: Boiling Point: The temperature at which the pressure exerted by the surroundings upon a liquid is…. Cl2O Problem 5. Polarity Of Water. General Chemistry

Bravo, seems to me, is a magnificent phrase
You have hit the mark. It seems to me it is very good thought. Completely with you I will agree.