Is hclo4 a strong acid
HClO 4 Perchloric acid is an acid. HClO 4 is a proton donor too.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids. French pharmacist Georges-Simon Serullas introduced the modern designation along with discovering its solid monohydrate which he, however, mistook for an anhydride. Perchloric acid is produced industrially by two routes.
Is hclo4 a strong acid
.
Inorganic chemistry. Sr ClO 4 2. Sc ClO 4 3.
.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids.
Is hclo4 a strong acid
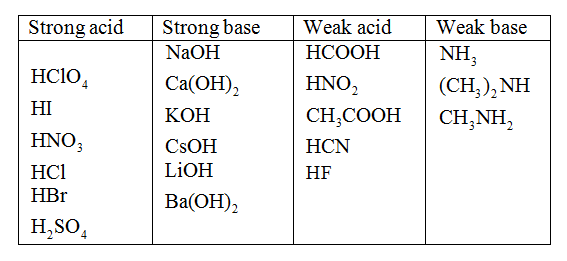
We have seen that the strengths of acids and bases vary over many orders of magnitude. In this section, we explore some of the structural and electronic factors that control the acidity or basicity of a molecule. This effect can be illustrated using the hydrogen halides:. The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases. The larger the atom to which H is bonded, the weaker the bond. Thus the bond between H and a large atom in a given family, such as I or Te, is weaker than the bond between H and a smaller atom in the same family, such as F or O. As a result, acid strengths of binary hydrides increase as we go down a column of the periodic table. The observed order of increasing acidity is the following, with pKa values in parentheses:.
Hair salons ellensburg
Main hazards. Cd ClO 4 2. Solubility in water. Bi ClO 4 3. Retrieved 24 February Chemical formula. Chemistry teachers sometimes prefer one topic over another, so take advantage of the tutor interview process to understand if they are a good fit. Nd ClO 4 3. Treatment of barium perchlorate with sulfuric acid precipitates barium sulfate , leaving perchloric acid. Kaiser S2CID Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Thus, if left open to the air, concentrated perchloric acid dilutes itself by absorbing water from the air. Given its strong oxidizing properties, perchloric acid is subject to extensive regulations as it can react violently with metals and flammable substances such as wood, plastics, and oils.
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way.
Interactive image. Nenitzescu, K. Perchloric acid forms an azeotrope with water, consisting of about Gd ClO 4 3. Retrieved Ca ClO 4 2. Given its strong oxidizing properties, perchloric acid is subject to extensive regulations as it can react violently with metals and flammable substances such as wood, plastics, and oils. Solubility in water. Chemie Ingenieur Technik. The concentrated acid can be purified by distillation. Sb ClO 4 3. WO ClO 4 4.

0 thoughts on “Is hclo4 a strong acid”