Is h2 polar or nonpolar
Are non-polar N 2H 2O 2 covalent bonds strong? The explanation of the strength of covalent bonds in N 2H 2O 2 is explained below:.
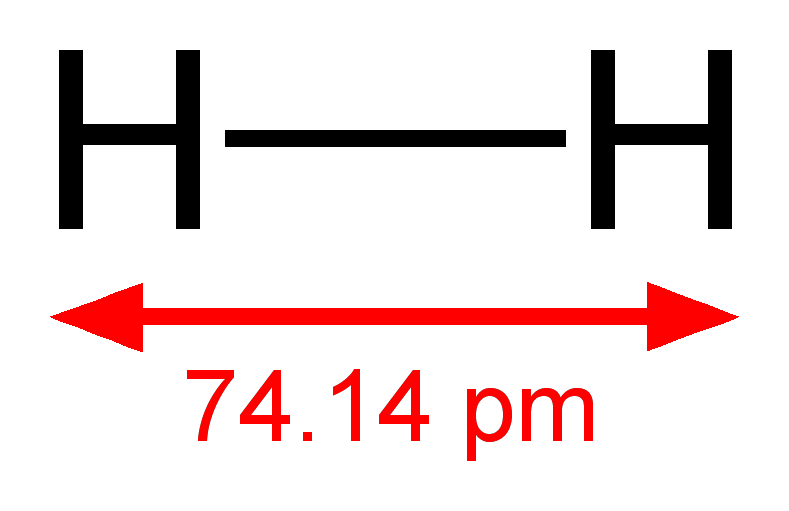
In a polar covalent bond, one atom is substantially more electronegative than the other, and strongly polarizes electron density towards itself, i. Now while the bond is still covalent, the more electronegative atom polarizes electron density A hydrogen atom has a certain electronegativity how much it pulls electrons to itself in a compound. Since each pull is equal and opposite, the electrons are pretty much distributed equally, meaning it is nonpolar. Apr 11,
Is h2 polar or nonpolar
For more option use Advanced Search. Electrons are shared differently in ionic and covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ionic bond analogy. The thief puppy has both bones i. The other puppy has lost its bone electron. The puppies are held together because of the electrostatic force caused by their charge difference. Non polar covalent bond analogy. Both puppies have an equal hold on both bones. Neither puppy has a charge; they are neutral. Polar covalent bond analogy. One puppy is able to pull more on the bones, but both puppies still have a hold on both bones. The polar covalent bonding of hydrogen and oxygen in water results in interesting behavior, suc.
The same would be true in the presence of a positively charged object; the water molecules turn so that the negative oxygen poles face the positive object.
.
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn't matter which direction the dipole moment vectors are pointing out or in.
Is h2 polar or nonpolar
Polar and nonpolar molecules are the two broad classes of molecules. Polarity describes the distribution of electrical charge around a molecule. Charge is evenly distributed in a nonpolar molecule, but unevenly distributed in a polar molecule. In other words, a polar molecule has regions of partial charge. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds , and how you can use polarity to predict which molecules will mix. Understanding and identifying polar and nonpolar chemical bonds makes it easier to understand polar molecules.
128 libre in kg
The hydrogen atom has a slightly positively charge because it cannot hold as tightly to the negative electron bones. Can a non polar molecule have polar covalent bonds? This document may be freely reproduced and distributed for non-profit educational purposes. For example, the polar compound methyl alcohol has a negative pole made of carbon and hydrogen and a positive pole made of oxygen and hydrogen see Fig. Water stream bending due to electrostatic force generated by rubbing a plastic comb on dry hair. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below:. Types of Covalent Bonds: Polar and Nonpolar. The other puppy has lost its bone electron. In a polar covalent bond, one atom is substantially more electronegative than the other, and strongly polarizes electron density towards itself, i. Neither puppy has a charge; they are neutral. Even though the electrons in hydrogen fluoride are shared, the fluorine side of a water molecule pulls harder on the negatively charged shared electrons and becomes negatively charged. Covalent bonds are soluble in non polar solution so will it be soluble in water? Standard XII Chemistry. See Fig.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
The fluorine atom acts as a slightly stronger puppy that pulls a bit harder on the shared electrons see Fig. Explanation: In a polar covalent bond, one atom is substantially more electronegative than the other, and strongly polarizes electron density towards itself, i. Some covalently bonded molecules, like chlorine gas Cl2 , equally share their electrons like two equally strong puppies each holding both bones. The polar covalent bonding of hydrogen and oxygen in water results in interesting behavior, suc. In part d , the diagram shows the relative size of the atoms, and the bonds are represented by the touching of the atoms. Partner Organizations. Symmetrical molecules are nonpolar. The hydrogen atom has a slightly positively charge because it cannot hold as tightly to the negative electron bones. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles - a positive charge on the hydrogen pole side and a negative charge on the oxygen pole side. One or more of these asymmetric atoms pulls electrons more strongly than the other atoms. How do atoms achieve stability in single covalent bonds?

0 thoughts on “Is h2 polar or nonpolar”