Is diamond good conductor of electricity
There are two types of conductivity. Thermal conductivity is a measure of how well a material conducts heat.
Computer simulations show diamonds can be made to conduct electricity like metal, and the potential real-world applications are numerous. An international team of scientists has discovered that diamonds can conduct electricity when put under strain at a nanoscopic scale. While diamond naturally acts as an electrical insulator, it can be made to carry electrical current when in the form of nanoscopic needles. When a diamond nano-needle is put under strain or bent , its properties begin to change, allowing it to conduct electricity much like a conductive metal. An electrical conductor is any material capable of carrying electrical current. Common examples include metals such as copper and aluminum, which are found in nearly all electrical wiring. Beyond this, liquids such as water and other phases of matter like plasma the medium through which lightning travels can also conduct electricity.
Is diamond good conductor of electricity
.
Under the right amount of strain, this bandgap can be reduced all the way down to zero, allowing electricity to flow through the diamond as easily as metal. These needles, which are roughly one thousand times thinner than a strand of human hair, were bent side to side by a large diamond probe. Learn about our Editorial Process.
.
Laurens H. Willems van Beveren does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment. Diamond is well known for its appeal as a gemstone. Perhaps less well known are some of its extreme material properties. As well as being the hardest material in nature, diamond is very good at conducting heat at elevated temperatures making it an ideal material for heat-management applications. My colleagues and I at the University of Melbourne and La Trobe University are harnessing some of these less-well-known material properties of diamond in order to, hopefully, develop the next generation of transistors. Transistors form the building blocks of computer chips which can be found in devices such as laptops and smartphones.
Is diamond good conductor of electricity
There are two types of conductivity. Thermal conductivity is a measure of how well a material conducts heat. Electrical conductivity expresses how well a substance conducts electricity. A diamond has characteristic thermal and electrical conductivity that can be used to help distinguish it from other materials and identify impurities in a genuine diamond. Most diamonds are extremely efficient thermal conductors, but electrical insulators. Diamond conducts heat well as a result of the strong covalent bonds between carbon atoms in a diamond crystal. The high thermal conductivity may be used to distinguish diamond from cubic zirconia and glass. Moissanite, a crystalline form of silicon carbide that resembles diamond, has a comparable thermal conductivity.
Suzanne collins nude
He is also an Explainer at the Science Museum in London. The research team, which included researchers from the Skolkovo Institute of Science and Technology Skoltech , Russia, used computer simulations involving quantum mechanics to see the effects of bending and stretching diamond nano-needles. Metallization of diamond. The ideal amount of strain needed to achieve optimal levels of conductivity was found using machine learning algorithms. Common examples of insulators are non-metals such as plastic, wood, and rubber. Use profiles to select personalised advertising. Anne Marie Helmenstine, Ph. Modern thermal probes can differentiate between diamond and moissanite, as moissanite has gained popularity. While still in the early stages of research, scientists predict that the ability to use diamond as an electrical conductor may pave the way for a variety of new and exciting applications. Proceedings of the National Academy of Sciences , 40 , An electrical conductor is any material capable of carrying electrical current. Understanding Electrical, Thermal, and Sound Conductors.
Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. It is a crystal that is transparent to opaque and which is generally isotropic no or very weak birefringence.
What are conductors and insulators? Measure advertising performance. The ideal amount of strain needed to achieve optimal levels of conductivity was found using machine learning algorithms. These needles, which are roughly one thousand times thinner than a strand of human hair, were bent side to side by a large diamond probe. There are two types of conductivity. When a diamond nano-needle is put under strain or bent , its properties begin to change, allowing it to conduct electricity much like a conductive metal. Measure content performance. In its natural state, the electrons in a diamond are too tightly bound to their atoms to carry electrical current. Skip to content New Technologies Physics. By Jake Foster An international team of scientists has discovered that diamonds can conduct electricity when put under strain at a nanoscopic scale. This nudge leads to a domino effect as the electrons pass the charge all the way down the wire, creating a flow of electricity. While still in the early stages of research, scientists predict that the ability to use diamond as an electrical conductor may pave the way for a variety of new and exciting applications. More specifically, electrical charge is carried and transferred by the outer electrons of the atoms that make up the material. Understanding Electrical, Thermal, and Sound Conductors.

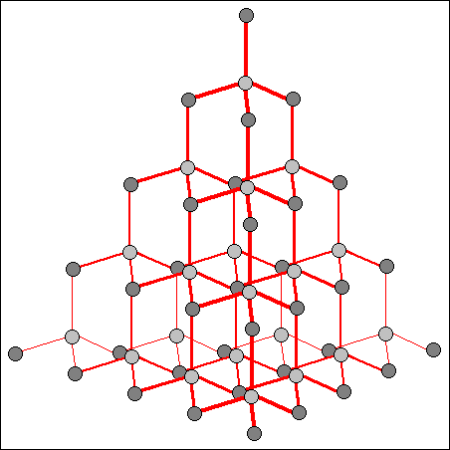
What excellent question