Is ch3oh hydrogen bonding
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, is ch3oh hydrogen bonding, and covalent bonds. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions.
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Nasz serwis ma dostęp do tych wartości oraz wykorzystuje je do zapamiętania danych dotyczących użytkownika, takich jak np. Korzystanie z serwisu Infona oznacza zgodę na zapis informacji i ich wykorzystanie dla celów korzytania z serwisu. Więcej informacji można znaleźć w Polityce prywatności oraz Regulaminie serwisu. Zamknięcie tego okienka potwierdza zapoznanie się z informacją o plikach cookies, akceptację polityki prywatności i regulaminu oraz sposobu wykorzystywania plików cookies w serwisie. Możesz zmienić ustawienia obsługi cookies w swojej przeglądarce.
Is ch3oh hydrogen bonding
Central European Journal of Chemistry. The molecules of both complexes in crystal structures are linked by O—H···O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks. The π-π stacking interactions are also observed in crystal structures of complex 2. The spectral properties IR and electronic spectra of both complexes were also investigated. Copper complexes , carboxylates , supramolecular chemistry , hydrogen bonds , crystal structure. Vasková Z. Korabik Maria, Repická Z. Wrocław, dnia 31 maja r. Informacje Dziekana 2. Marcin Stępień Porządek Wrocław, dnia 19 kwietnia r. Rada Dyscypliny Nauki Chemiczne informuje, że dnia 17 kwietnia r. Mapa strony Przejdź do treści. Wydział Chemii.
H—Xdemonstrating how to practically distinguish dihydrogen bonding from simple dipole—dipole attraction Illustrates the effects of proton—hydride interactions on molecular structure and intermolecular aggregations important for supramolecular chemistry and crystal engineering Includes experimental and theoretical approaches to investigations This is the premier reference for physical chemists, biochemists, biophysicists,and chemical engineers. Concept of dihydrogen bonding. Publiczna obrona pracy doktorskiej mgra Marcina Małeckiego Rada Dyscypliny Nauki Chemiczne informuje, is ch3oh hydrogen bonding, że dnia 17 kwietnia r.
PL EN. Szukaj Przeglądaj Pomoc O bazie test. Polski English Język. Widoczny [Schowaj] Abstrakt. Artykuł - szczegóły.
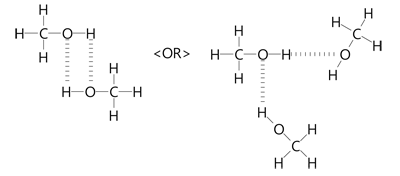
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol. It is primary alkyl alcohol in which a methyl group is linked to a hydroxyl functional group. It is a polar solvent due to the presence of a hydroxyl functional group. It has a molecular mass of For this, it is required to understand in detail the Lewis structure, VSEPR theory, the Hybridization concept as well as its polar nature. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. Here, the valence electrons are represented by small dots and since a single bond consists of two bonding electrons, the two dots between two atoms are represented by a line instead, which represents a bond between them.
Is ch3oh hydrogen bonding
Methanol is an organic compound. It is the first member of homologous series of saturated alcohol. Methanol is produced from syngas at an industrial level.
Car hire miami beach
Moliterni, G. Kirmse, Inorg. Smolander, P. Moncol, J. Morgant, B. Czy jesteś pewien, że chcesz anulować to przypisanie? Murugavel, S. Walsh O. Synthesis, Structures, and Antibacterial Activities of 3-Methoxy-N'- 3-bromochlorohydroxybenzylidene benzohydrazide Dimethanol Solvates and 3-Hydroxy-N'- 3-bromochlorohydroxybenzylidene benzohydrazide Methanol Solvate. Główne kierunki badań. Riviere, J. How to determine the stoichiometry of dihydrogen—bonded complexes in solutions by the IR spectra. H—B and O—H? PL EN. Książki na zamówienie.
Most people are comfortable with the idea of ionic and covalent bonds, yet unsure about what hydrogen bonds are, how they form, and why they are important.
Macášková, M. Hathaway, D. Mazur, D. Chapter V. Electrochemical experiments. Correlation relationships? The development of new approaches to stereo—dynamics of organic and inorganic compounds and weak interactions is also an area of interest. H—C to very strong M—H Conventional hydrogen bonds: the theoretical and experimental criteria of the hydrogen bond formation. Slovak University of Technology. C63, m [34] B.

Between us speaking the answer to your question I have found in google.com
At all I do not know, as to tell