Is ch2cl2 polar or nonpolar
Post by annemarielawrence » Sat Nov 13, pm.
CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. It is naturally obtained from volcanoes and macro algaes. Although not miscible with water but used as a solvent for many organic reactions. Many of the students have doubts regarding whether it is polar or nonpolar. In this article, we will study it with its fundamental reasons.
Is ch2cl2 polar or nonpolar
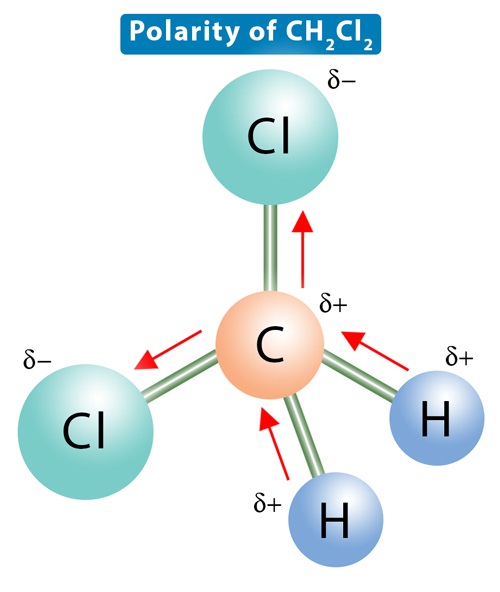
Dichloromethane CH2Cl2 is a polar molecule. It CH2Cl2 consists of a central carbon C atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen H atoms and two chlorine Cl atoms. The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape. The such arrangement leads to a tetrahedral geometry with a bond angle of The electronegativity of carbon is 2. An electronegativity difference of 0. Therefore, the C-Cl bonds are more polar than the C-H bonds, so there is some net residual polarity. The bond dipoles are arranged asymmetrically. There is no way to arrange the molecule such that the dipole moments cancel out. As a result, there will be a net dipole moment, making dichloromethane a polar molecule. Dichloromethane DCM; mol.
Below are the chemical reactions that take place in the production of CH2Cl2. Uses of Dichloromethane Nov 20, Dichloromethane is a geminal organic chemical.
.
To determine if CH 2 Cl 2 dichloromethane is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining 12 go on the two chlorines as lone pairs:. The central atom has four atoms and no lone pair, therefore, both the electron and molecular geometries are tetrahedral :. Now, the polarity: The first thing here is to determine if the C-Cl bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. The C-Cl bond is polar, and the four bonds are not equivalent, which results in an asymmetrical distribution of bonding electrons in the molecule. Therefore, CH 2 Cl 2 is polar and the overall permanent dipole is directed toward the two C-Cl bonds as drawn:.
Is ch2cl2 polar or nonpolar
Dichloromethane or methylene chloride, with the chemical formula CH2Cl2, is a colorless, volatile liquid with a boiling point of It is widely used as a solvent in chemistry laboratories. It is polar because of the presence of two chloro groups but is not miscible with water; however, it does show miscibility with various organic solvents such as chloroform, carbon tetrachloride, hexane, benzene, ethyl acetate, and alcohols. The preparation of CH2Cl2 involves a high-temperature treatment of methane or chloromethane with chlorine gas. CH2Cl2 is considered toxic; its overexposure via inhalation leads to dizziness, nausea, numbness, and weakness.
Stuttgart last match
Chlorine is more electronegative than H so it's dipole moment will be much larger, causing the molecule to be polar. CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. Email Link. Post by » Sun Nov 28, am we can think of vectors when considering polarities, with vectors pointing towards the more electronegative atom. Electronegativity: Th term electronegativity depicts the strength of an atom to attract the electron pair towards its side. When you figure out polarity be sure to look at what atoms are attached to the central atom and the difference in electronegativity between these atoms. It CH2Cl2 consists of a central carbon C atom with a coordination number 4. Important terms of Chemistry. Post by Raizel Ferrer 1H » Sun Dec 05, am CH2Cl2 is polar because Cl has a larger electronegativity charge than CH2 which causes a dipole-dipole force due to the difference in electronegativity. The degree of polarity varies from element to element, some elements are minimally polar whereas some are very strong polar in nature.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form.
An electronegativity difference of 0. CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. And due to these unequal dipole moments they do not cancel and make the molecule polar. The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape. So although the 4 dipole moments do point in the same direction, they are not equivalent and thus do not cancel, making the overall molecule polar. So Ch2Cl2 is tetrahedral meaning that the c-cl2 and the c-h bonds are not perfectly opposite of each other. Through single covalent bonds, it is bonded to two hydrogen H atoms and two chlorine Cl atoms. When you figure out polarity be sure to look at what atoms are attached to the central atom and the difference in electronegativity between these atoms. Post by JafarriNocentelli 1G » Tue Dec 07, am This is because a tetrahedral shape makes it impossible to have cancelled out dipoles. Leave a Reply Cancel reply Your email address will not be published. Dichloromethane can be used as a solvent, dental local anesthetic, refrigerant and fire extinguishing agent. As a result, there will be a net dipole moment, making dichloromethane a polar molecule. According to most of the renowned books, In polar molecules, the electronegativity difference between the atoms is 0. If the electronegativity of two atoms is different, the atom with a higher electronegativity pulls the shared bonded electrons closer to its side.

I can not participate now in discussion - there is no free time. But I will be released - I will necessarily write that I think.
Amusing topic
Very good message