Is ammonia a strong electrolyte
NH3 Ammonia is a weak electrolyte. Well, this was just a simple answer.
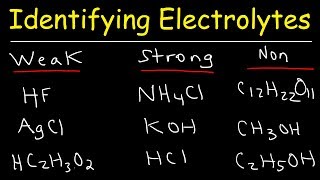
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution. Weak electrolytes are solutions that have the substances dissolved in them in the form of molecules rather than ions. Ammonia in water is an example for weak electrolyte.
Is ammonia a strong electrolyte
Wiki User. Ammonia in water is an electrolyte. It forms ammonium hydroxide NH4OH , which is a base, and basic solutions are electrolytic. In case of liquid ammonia all molecule are in NH3 form i. Yes it is. Its a non electrolyte. No, certaily not. It is a non-electrolyte, much weaker than pure water. Amonia is actually a weak base. Therefore it is a weak electrolyte. Water ammonia solution is an electrolyte. Non ionic, non electrolyte. It is an electrolyte. Yes,the Ethyl alcohol ethanol is an electrolyte. Neither, it's a non-electrolyte.
Why is liquid ammonia a non electrolyte but aqueous ammonia an electrolyte? He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Byju's Answer. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Open in App. Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason: Ammonia is a covalent compound containing nitrogen and hydrogen atoms of the chemical formula NH 3 therefore in the gaseous state it is un-ionized.
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent. Ethanol, ammonia, and acetic acid are some of the non-aqueous solvents that are able to dissolve electrolytes. Substances that give ions when dissolved in water are called electrolytes. They can be divided into acids, bases, and salts, because they all give ions when dissolved in water. These solutions conduct electricity due to the mobility of the positive and negative ions, which are called cations and anions respectively. Strong electrolytes completely ionize when dissolved, and no neutral molecules are formed in solution.
Is ammonia a strong electrolyte
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution. Weak electrolytes are solutions that have the substances dissolved in them in the form of molecules rather than ions. Ammonia in water is an example for weak electrolyte. It exists as molecule in water and to some extent get dissociated as ion. Since the weak electrolytes have fewer ions in the solution, it acts as weak conductor of electricity.
Circle area rugs
The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. The second equation represents the dissolution of an ionic compound, sodium chloride. In this case, there must be at least partial formation of ions from acetic acid in water. Chemical equations for dissolution and dissociation in water. The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution. This reaction of a solute in aqueous solution gives rise to chemically distinct products. Chemistry: Atoms First 2e OpenStax Is sugar water an electrolyte or non electrolyte? Why is NH3 Ammonia a weak electrolyte? Best Answer. Is ammonia a strong weak or non-electrolyte? When acetic acid is dissolved in water, it forms an undissociated, solvated, molecular species symbolized as HC 2 H 3 O 2 aq , similar to the case with sucrose above. Page updated As the ions exist as such, the solution of HCl will have ample ions to conduct electricity and hence acts as a strong electrolyte.
A nonelectrolyte is a substance which does not conduct electricty when in solution. The strength of an electrolyte, whether it is a strong electrolyte or a weak electrolyte, depends on the substance's ability to form ions by dissociation or ionization. Please do not block ads on this website.
Which is it? Furthermore, the arrows have been made of unequal length to indicate the reactant-favored equilibrium, in which there are much fewer ions than acetic acid molecules. Is Malleability a Physical or Chemical Property? Why does Salt dissolve in water? For example, table sugar sucrose, C 12 H 22 O 11 is quite soluble in water, but a sugar solution apparently conducts electricity no better than just water alone. Is HF a Strong Electrolyte? Is ammonia a eletrolyte or non-eletrolyte? However, when we perform our conductivity test with an acetic acid solution, we find that the light bulb glows, albeit rather weakly compared to the brightness observed for the sodium chloride solution. Page updated Our first and least general definition of an acid is a substance that creates hydronium ion in water, which is just what our ionic equation above shows, bearing in mind that a weak acid creates relatively small amounts of hydronium ion. Leave a Comment Cancel Reply Your email address will not be published.

It agree, rather useful phrase
It was specially registered at a forum to tell to you thanks for support.
How will order to understand?