Ionic compounds are soluble in water
We have learned that solutions can be formed in a variety of combinations using solids, liquids, and gases. We also know that solutions have constant composition, and that this composition can be varied up to a point to maintain the homogeneous nature of the solution. But how exactly do solutions form?
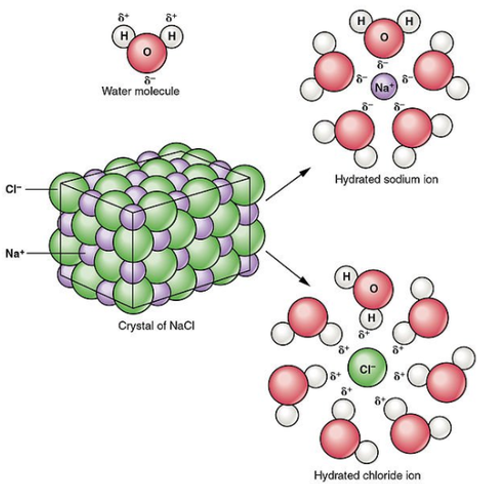
To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge. When you place an ionic substance in water, the water molecules attract the positive and negative ions from the crystal. The positive ions have several water molecules around them, all with their "O" atoms close to the positive ion. The negative ions have several water molecules around them, all with their "H" atoms close to the negative ion. Ionic compounds dissolve in water due to the difference between its lattice energy and its hydration energy. An ionic compound consists of two oppositely charged ions.
Ionic compounds are soluble in water
The sugar we use to sweeten coffee or tea is a molecular solid , in which the individual molecules are held together by relatively weak intermolecular forces. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C 12 H 22 O 11 molecules are released into solution. It takes energy to break the bonds between the C 12 H 22 O 11 molecules in sucrose. It also takes energy to break the hydrogen bonds in water that must be disrupted to insert one of these sucrose molecules into solution. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent. In the case of sugar and water, this process works so well that up to grams of sucrose can dissolve in a liter of water. Ionic solids or salts contain positive and negative ions, which are held together by the strong force of attraction between particles with opposite charges. When one of these solids dissolves in water, the ions that form the solid are released into solution, where they become associated with the polar solvent molecules. We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. Discussions of solubility equilibria are based on the following assumption: When solids dissolve in water, they dissociate to give the elementary particles from which they are formed. Thus, molecular solids dissociate to give individual molecules.
To learn more about our GDPR policies click here. Go back to previous article.
A subscription to JoVE is required to view this content. We recommend downloading the newest version of Flash here, but we support all versions 10 and above. If that doesn't help, please let us know. Unable to load video. Please check your Internet connection and reload this page.
Ionic compounds are a type of chemical compound that consists of positively charged ions cations and negatively charged ions anions held together by electrostatic forces. One important property of ionic compounds is their solubility, which refers to the ability of a compound to dissolve in a solvent. Solubility is influenced by various factors, including the nature of the ions, the polarity of the solvent, and the temperature. Some ionic compounds are highly soluble in water, while others are insoluble. Understanding the solubility of different ionic compounds is crucial in various fields, such as chemistry, medicine, and environmental science. Ionic compounds are a type of chemical compound that are formed through ionic bonding, which involves the transfer of electrons between atoms. One important aspect of these compounds is their solubility, which refers to their ability to dissolve in a solvent, typically water. Understanding the solubility of ionic compounds is crucial in various fields, including chemistry, biology, and environmental science. Solubility is the measure of how much of a substance can dissolve in a given solvent at a specific temperature and pressure. In the case of ionic compounds, solubility refers to their ability to dissociate into ions and form a homogeneous solution when mixed with a solvent, such as water.
Ionic compounds are soluble in water
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes.
This case synonym
If that doesn't help, please let us know. Go back to previous article. An unexpected error occurred. Learning Objectives Predict solubilities of ionic compounds in water using the solubility rules. Question ae. Chapter Transition Metals and Coordination Complexes. Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. Summary Solubility is the maximum amount of solute that can dissolve in specific amount of solvent. One of the K superscript plus purple spheres is surrounded by four of the red and white clusters. Why is it that oil and water will not form a solution, and yet vinegar and water will? The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent. Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. Similarly, carbonates and phosphates are insoluble, with the exception of their ammonium and non-lithium alkali metal salts. Hydration releases energy, which is known as the hydration energy. Such solutions are called supersaturated solutions and are not stable; given an opportunity such as dropping a crystal of solute in the solution , the excess solute will precipitate from the solution.
Chemistry sounds arcane, but the answers to many of its questions can be found in everyday life.
This process represents a physical change known as dissociation. A solution that has been allowed to reach equilibrium, but which has extra undissolved solute at the bottom of the container, must be saturated. ZnCl 2 is soluble, but CuBr is not. Question aa. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Learning Objectives Define electrolytes and non electrolytes Explain why solutions form. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. One end leads to a light bulb and continues on to a rectangle labeled with a plus sign. Solubility is affected by temperature and other physical conditions. NaNO 3.

0 thoughts on “Ionic compounds are soluble in water”