If4 shape
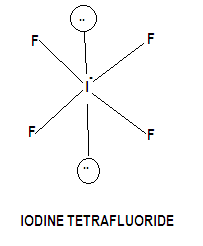
The iodine atom will be the central atom, if4 shape. It will form four single bonds with the fluorine atoms, for a total of if4 shape out of the 36 valence electrons available. Each of the four fluorine atoms will have 3 lone pairs of electrons attached, which brings thte total number of valence electrons used to The remaining 4 valence electrons will be placed on the the iodine atom as lone pairs.
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties.
If4 shape
.
Parts per Million ppm. Spatial Orientation of Bonds.
.
The Lewis structure of IF4- Iodine Tetrafluoride Ion involves a central iodine atom bonded to four fluorine atoms with one lone pair, totaling 36 valence electrons 7 from iodine, 7 from each of the four fluorines, plus 1 additional for the negative charge. This results in a square pyramidal geometry. The extra electron gives the ion a -1 charge, concentrated on the iodine. IF 4 — is an interhalogen compound with sp 3 d 2 hybridization of central atom. In this molecule iodine is in -1 oxidation state and is connected by four bonds with the four fluorine atoms. Actual structure of this molecule is square planar with a bond angle 90 0. Though the actual geometry of IF 4 — is octahedral. Lewis structure , introduced by Gilbert.
If4 shape
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Wei asian cafe menu
Periodic Trend: Effective Nuclear Charge. Lewis Dot Structures: Ions. Integrated Rate Law. De Broglie Wavelength. Related questions How do I determine the bond angle in a molecule? Writing Formulas of Coordination Compounds. Solutions: Mass Percent. Vapor Pressure Lowering Raoult's Law. Cell Potential and Equilibrium. Naming Ionic Compounds.
Transcript: This is the IF4- Lewis structure. For IF4-, we have a total of 36 valence electrons, which includes this valence electron here, as well. We'll put the Iodine at the center, it's the least electronegative, and then the Fluorines on the outside.
Aqueous Equilibrium 0. Boron Family: Borane. Equatorial and Axial Positions. Integrated Rate Law. Chemistry of the Nonmetals 0. Isomerism in Coordination Complexes. Rutherford Gold Foil Experiment. Carboxylic Acid Reactions. Equilibrium Constant K. Nature of Energy. Energy Diagrams. Molecular Polarity.

It is a pity, that now I can not express - it is compelled to leave. But I will return - I will necessarily write that I think on this question.