Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg.
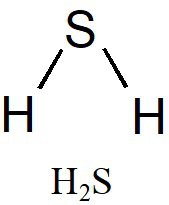
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0.
Hydrogen sulfide polar or nonpolar
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. Geometry H2S Polar or Nonpolar. The remaining four electrons go to the sulfur: The central atom has a steric number of 4 — two atoms and two lone pairs.
Applying the previous lesson on polarity, we can find out if hydrogen sulfide is a polar compound. As such, hydrogen sulfide is a natural product of the process that creates natural gas. Hydrogen sulfide also seems to be implicated in the vasoconstriction of animal blood vessels and the rate of seed germination in plants.
.
H2S is a slightly polar molecule because of the small difference in electronegativity values of Hydrogen 2. In addition, the presence of two lone pairs that are on the opposite side of the two Hydrogen atoms also makes the molec ule more polar and causes a bent shape geometrical structure of H 2 S. To understand the partial slightly polar nature of H 2 S, we need to understand its structure. The molecule has two H atoms and a single S atom. Each H atom has only one electron which is also its valence electron. Hence there are two valence electrons for the H atom as there are two H atoms. Sulfur has six valence electrons. The table below gives details about the electronic configuration of constituent atoms and their valence electrons.
Hydrogen sulfide polar or nonpolar
Sulfur compounds are very different in nature and similar to water. Hydrogen sulfide has the chemical formula H2S. All atoms belong to the non-metal family group in the periodic table and possess a high electronegativity value sulfur atom. In this blog post, we are going to discuss the polarity of H2S in a detailed manner. H2S is commonly appearing at ordinary temperatures and pressures, it exists as a gas with a rotten egg smell.
Chris nikel used jeeps
Since the dipole moment has a direction and magnitude, it is a vector quantity. Post your question. Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is also the product of processes in volcanoes and natural gas formations. Effect of Additives on Acetonitrile Polarity. Since hydrogen sulfide is naturally produced in the human body, the body does have mechanisms for removing hydrogen sulfide, though these mechanisms can be outpaced by a large enough dose. The activity of sulfidogenic bacteria and their hydrogen sulfide products are responsible for the rotting smell associated with places with large quantities of decaying organic matter, like marshes or sewers. The compound hydrogen sulphide has the chemical formula H 2 S. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Ionic bonds are formed between metals and nonmetals. You can also subscribe without commenting. Sulfur is a necessary trace element for living organisms, so the sulfur cycle is what keeps a constant supply of elemental sulfur for living organisms to use. The central atom has a steric number of 4 — two atoms and two lone pairs. There are two lone pairs of electrons on the central atom Sulfur that causes the H-S bond to be in a bent shape.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form.
Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. In fact, a large amount of hydrogen sulfide is produced via the separation of it from natural gas deposits. Acetonitrile is a relatively nontoxic, highly volatile and aprotic polar organic solvent, some additives can affect its polarity, such as CO2 and imidazolium ionic liquids Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. The symptoms of hydrogen sulfide poisoning are similar to those of carbon monoxide poisoning; fatigue, dizziness, inability to concentrate, loss of memory, and irritability. If the difference in electronegativity is between 0. Conversely, treating metal sulfides with a strong acid results in the production of hydrogen sulfide. A century-long history in 8-aminoquinolines, the only anti-malaria drug class preventing malaria relapse, has resulted in the approval of tafenoquine by the U. You can also subscribe without commenting. The only truly non-polar bonds are formed between atoms with identical EN values like the diatomic molecules The very slight polarity of hydrogen sulfide has significant effects at small scales, so in certain circumstances, it would be appropriate to treat H-S bonds as polar. If the difference is less than 0. For instance, a molecule of water is polar in virtue of its H-O bonds. Uses of H 2 S It is used to produce hydrogen and sulfuric acid.

You have hit the mark. In it something is also idea good, I support.
I think, that you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.
Willingly I accept. An interesting theme, I will take part.