Hydrogen bromide lewis dot structure
Wiki User. The electron dot formula for a monoatomic hydrogen is H.
Wiki User. HBr has a dipole. HBr is a strong acid. HBr has an ionic bond. The Lewis dot structure for hydrogen bromide HBr consists of a single covalent bond between the hydrogen atom and the bromine atom. So, there is one single covalent bond in the Lewis dot structure of HBr.
Hydrogen bromide lewis dot structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms. Total electron pairs are determined by dividing the number total valence electrons by two.
Continue Learning about Chemistry. Trending Questions.
.
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide HBr. It showcases the connectivity between hydrogen H and bromine Br atoms, giving us a visual representation of their covalent bond. Determine the Total Valence Electrons. Arrange Atoms. The HBr molecule consists of only two atoms, so you can choose any atom as the central atom. Connect hydrogen and bromine with a single bond: H-Br. Remember : If hydrogen is present in the molecule, always place the hydrogen atoms on the outside. Place the remaining electrons around the atoms.
Hydrogen bromide lewis dot structure
Transcript: Hi, this is Dr. Let's do the Lewis structure for HBr, hydrobromic acid. On the periodic table, Hydrogen is in group 1, so it has 1 valence electron, and Bromine is in group 7, sometimes called 17, it has 7 valence electrons; for a total of 8 valence electrons. We draw our H, we draw our Br, and we have 8 valence electrons. So let's do this: let's put 2 right here. That bonds Hydrogen and Bromine together; 4, 6, 8.
300000 inr to cad
What is a synonym for Lewis diagram? Still have questions? Continue Learning about Chemistry. How many single covalent bonds down at is the Lewis dot structure for hydrogen bromide? Find more answers Ask your question. Because there are no charges on atoms, no need to worry about reducing charges as a step of obtaining the best lewis structure. The electron dot formula for a monoatomic hydrogen is H. Also, remember that HBr is a molecule which does not have a charge. Please note the parentheses above are for clarification and are not part of the electron dot diagram. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. What is uranium Lewis dot number? What is the Lewis dot diagram for hydrogen and chlorine? How to draw the internal structure of a leaf? What is the system used to represent the valence electrons around the chemical symbol of an element? What kind of bond is HBr?
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
More answers. Previously Viewed. So, there is one single covalent bond in the Lewis dot structure of HBr. How do you draw the Lewis dot diagram for hydrogen chloride? Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms. HBr is a very easy lewis structure to draw due to its simplicity. Study now See answer 1. However, elemental hydrogen is diatomic, so most hydrogen atoms would be found as H:H. HBr lewis structure There are only one hydrogen atom and one bromine atom in HBr molecule. What is the Lewis dot structure for Ce? There are only one hydrogen atom and one bromine atom in HBr molecule. Continue Learning about Earth Science. Also, remember that HBr is a molecule which does not have a charge.

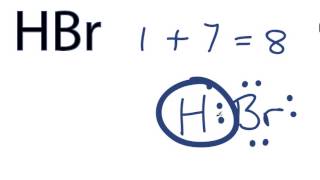
I am final, I am sorry, but it does not approach me. I will search further.