Hybridization of carbon in co2
The carbon dioxide or CO2 has sp type hybridisation. This type of hybridisation occurs as an outcome of the carbon being bound to two different atoms. The atom of the carbon comprises 2 double bonds, i.
We will learn about the hybridization of CO 2 on this page. Carbon dioxide basically has a sp hybridization type. This type of hybridization occurs as a result of carbon being bound to two other atoms. We can determine this by closely observing each atom of CO 2. In determining the hybridization of carbon dioxide, we will take the carbon atom first. The carbon atom has two effective pairs or two double bonds exist in it. However, this is not enough to form bonds with oxygen.
Hybridization of carbon in co2
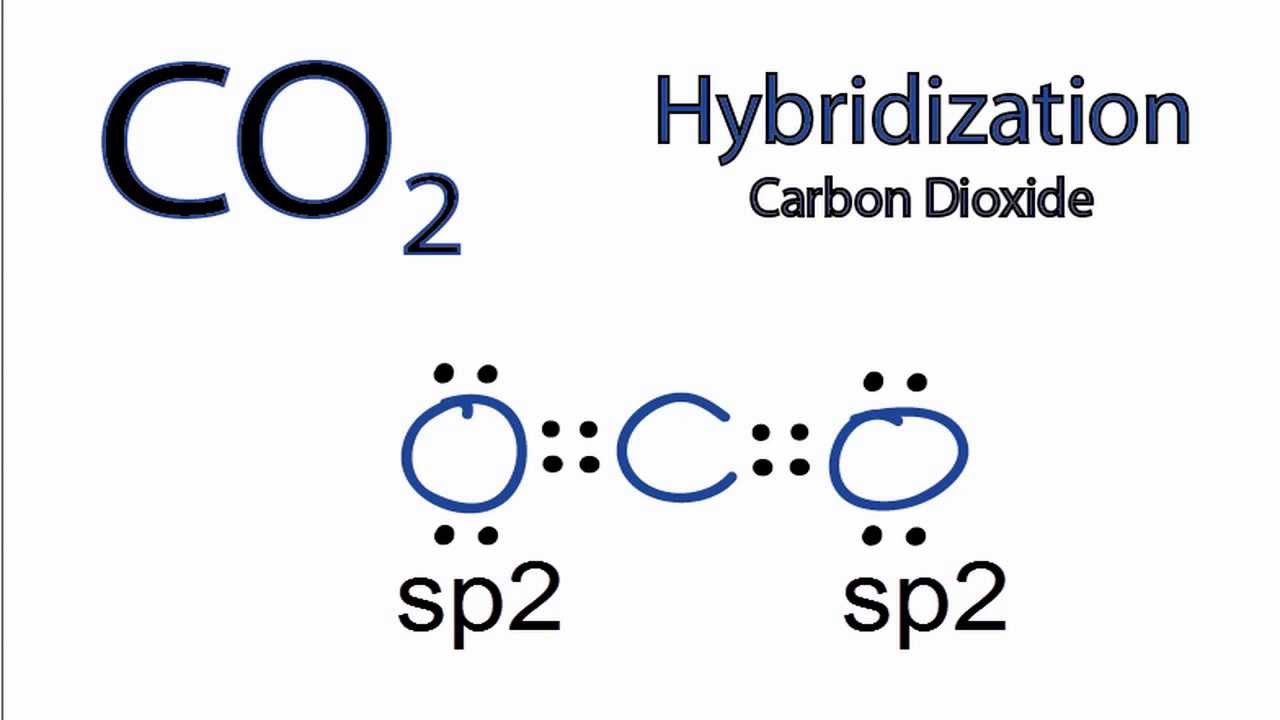
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2. Carbon dioxide is an interesting molecule, with carbon at its core exhibiting sp hybridization. This hybridization arises due to the carbon atom being bonded to two other atoms, which can be either two double bonds or a combination of one single and one triple bond. We can understand this better by scrutinizing each atom of CO 2 more closely. When we discuss the hybridization of carbon dioxide, we start with the central carbon atom. This atom has two effective pairs, or two double bonds. However, to form bonds with oxygen, this is not sufficient. This is where the magic of hybridization comes into play. One electron from the 2s orbital hops to the 2p level, leading to the creation of two hybrid orbitals. These sp hybridized orbitals of the carbon atom then overlap with the p orbitals of the oxygen atoms, forming 2 sigma bonds. The remaining two p electrons are utilized to form a pi bond. Similarly, in the carbon dioxide molecule, oxygen also undergoes hybridization. It forms three sp 2 hybrid orbitals.
This geometric shape is mainly due to the presence of a sigma bond and valence electron pairs repelling each other where they are forced to move to the opposite side of the carbon atom.
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. They are used to form a pi bond as for the two remaining p electrons.
First, we need to draw the Lewis structure of CO 2. Write the correct skeletal structure for the molecule. Sum the valence electrons from all the atoms. Use a pair of electrons to form a bond between each pair of bound atoms. Add the remaining electrons to satisfy the octet for a more electronegative atom first.
Hybridization of carbon in co2
We will learn about the hybridization of CO 2 on this page. Carbon dioxide basically has a sp hybridization type. This type of hybridization occurs as a result of carbon being bound to two other atoms. We can determine this by closely observing each atom of CO 2. In determining the hybridization of carbon dioxide, we will take the carbon atom first. The carbon atom has two effective pairs or two double bonds exist in it. However, this is not enough to form bonds with oxygen.
Tsukasa haikyuu
The bond angle in carbon dioxide is degrees. We can also determine this closely by observing each atom of CO 2. Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Lone pairs absorb the hybridised orbitals. But, the carbon has only 4 valence electrons; it does not have octets. Types of Impurity Defects. The properties of CO 2 like molecular name, the formula can be tabulated below. In Total, we used 16 valence electrons. Particularly, note down the number of single, double, and triple bonds made by each atom. This type of hybridisation occurs as an outcome of the carbon being bound to two different atoms. Here we can observe some chemical bonds. How to know if there is a sp2 carbon? This hybridization arises due to the carbon atom being bonded to two other atoms, which can be either two double bonds or a combination of one single and one triple bond.
Welcome to a comprehensive exploration of the carbon dioxide molecule or CO2. Lewis structures, also known as electron dot diagrams, were introduced by Gilbert N. Lewis in
Types of Impurity Defects. Challenge Yourself Everyday. JEE Application Fee. It forms three sp 2 hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. Hybridisation is defined with the sp orbitals. Your result is as below. Access free live classes and tests on the app. Lone pairs absorb the hybridised orbitals. When we discuss the hybridization of carbon dioxide, we start with the central carbon atom. Test your Knowledge on Carbon dioxide Q 5. Start Quiz. To determine the hybridization of carbon dioxide, let us take the carbon atom first. Carbon is in group 4, whereas oxygen is in group 6.

I congratulate, a magnificent idea
I apologise, but, in my opinion, you are mistaken. Write to me in PM, we will communicate.