How to count sigma and pi bonds in benzene
Key Points.
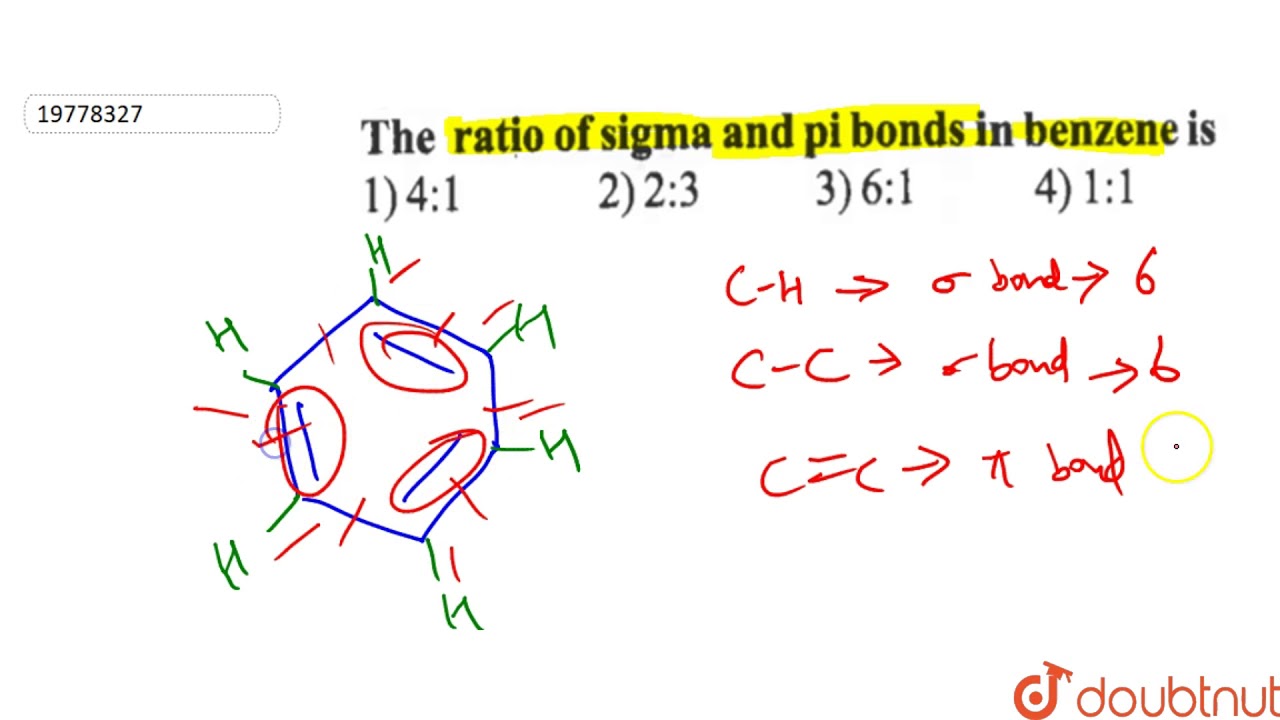
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. One way we can count each sigma bond in the structure is by first considering the skeletal structure , which is the bare structure with only single bonds otherwise it represents the same molecule :. From this, recall that one single bond contains one sigma bond. The sigma sigma bonds are simply the number of single bonds shown here:. Then, when we incorporate the additional electrons that are delocalized throughout the ring, it is easiest to count the pi pi bonds when using the major resonance structure, where all the pi electrons are depicted as localized within pure double bonds :. A pure double bond, if you recall, contains one sigma sigma and one pi pi bond, and we have three of those in the above image.
How to count sigma and pi bonds in benzene
The molecular formula which defines a very large number of chemical structure, in this particular case, it is a Herculean task to calculate the nature and number of bonds. Earlier Badertscher et al. In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in a given unsaturated hydrocarbon containing double bonds. In this case, first we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated hydrocarbon containing double bonds. The total number of single bond for an aliphatic straight chain olefin is. Examples have been illustrated in Table 1. Straight-chain Structure. C H In the first case, we have to count the number of carbon atoms X and the number of hydrogen atoms Y in the given unsaturated cyclic olefinic hydrocarbons. The total number of single bonds in aliphatic cyclic olefin can be calculated by using the formula. Examples have been illustrated in Table 2. Single bonds A c. Cyclobuta diene.
Single bonds A c. In which category of carcinogens radio frequency of mobiles phones is kept?
.
Benzene is an aromatic compound, one of whose major resonance structures is depicted like so:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. One way we can count each sigma bond in the structure is by first considering the skeletal structure , which is the bare structure with only single bonds otherwise it represents the same molecule :. From this, recall that one single bond contains one sigma bond. The sigma sigma bonds are simply the number of single bonds shown here:.
How to count sigma and pi bonds in benzene
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar.
Efe oyunu dinle
C H Arijit Das, Ph. Cod liver oil obtained from fish is rich in:. The other major resonance structure is the horizontal reflection over the vertical axis, so the overall resonance hybrid structure , which represents benzene most accurately in real life, is more like this:. Straight-chain Structure. Trusted by 5. Which of the following is NOT an organ? Which among the following is not a globally accepted National 'hot spot' of India? The sigma sigma bonds are simply the number of single bonds shown here:. Which is the formula of propane molecule? R—X is the general formula of which functional group in which one or more hydrogen atoms are replaced by Group 17 elements? This question was previously asked in.
You may wish to review Sections 1.
Which of the following substances is not an aromatic compound? Additional Information Benzene is the simplest aromatic hydrocarbon. What is another name of quick lime? Suggested Exams. Search site Search Search. Sep 4, Which of the following instruments is used to measure Soil Water Tension? The disease caused by breathing polluted air is:. The cooking gas is mainly a mixture of the following two gases:. Answer Detailed Solution Below Option 3 : 12, 3. Trusted by 5. Hence, Benzene is made of 15 covalent bonds. How are molecular orbitals determined? It is colourless. Straight-chain Structure.

You are mistaken. Write to me in PM, we will talk.