How many valence electrons in o
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
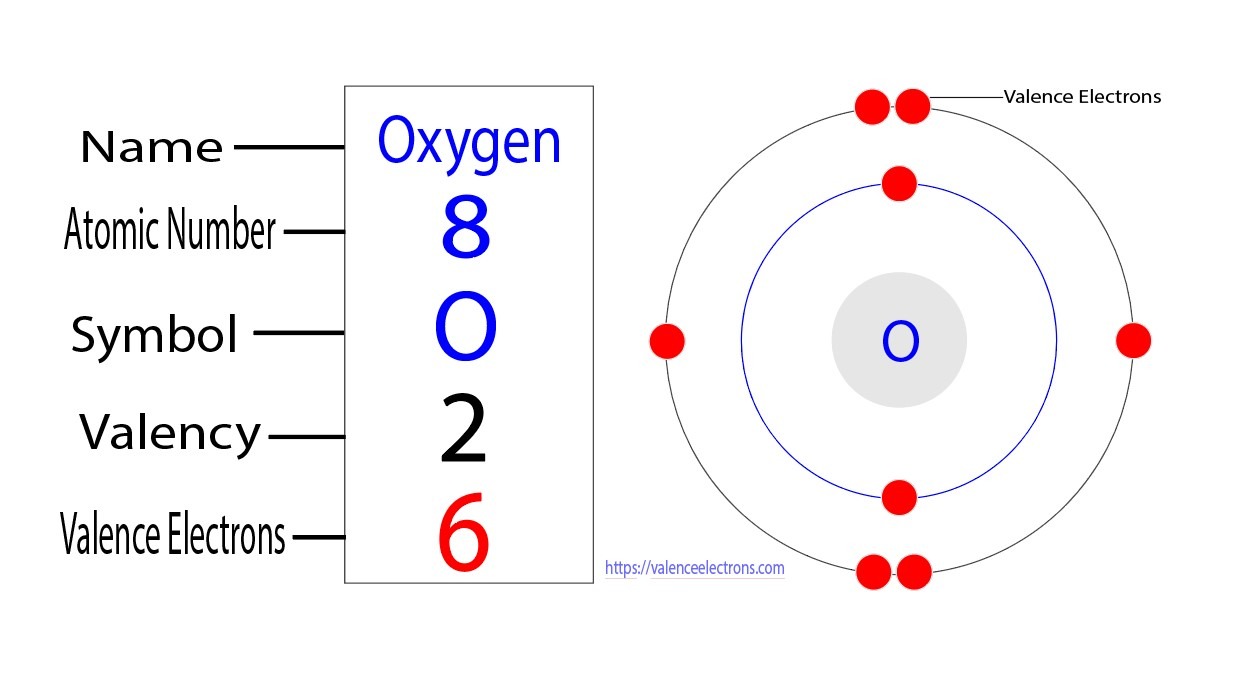
Oxygen has 6 valence electrons. A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements columns 1, 2, , the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc. How many valence electrons does oxygen have?
How many valence electrons in o
Key Points. Additional Information. Last updated on Feb 7, Along with the official notification, the apply online link will also be opened. Candidates will be able to apply online until 10th July, This year, SSC completely changed the exam pattern. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams.
The disease caused by breathing polluted air is:. BPSC Asst. ESIC Stenographer.
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons. How many valence electrons does O have?
Oxygen has six valence electrons , along with the other elements in group This means that it's outer energy level contains six valence electrons. If it is in group 1 or 2, the group number is the number of valence electrons. If it is in group , subtract If it is in the middle section transition metals, Lanthanoids, or Actinoids , the rules are not so clear-cut. Luckily Oxygen O is in group 16, so it has 6 valence electrons. How many valence electrons does O have? Oct 30, Explanation: This means that it's outer energy level contains six valence electrons.
How many valence electrons in o
The standard atomic mass of oxygen is Oxygen participates in the formation of bonds through valence electrons. Oxygen is a non-metallic element.
Law and order svu reddit
Oxygen O is a nonmetallic chemical element of Group 16 VIa, or the oxygen group of the periodic table. West Bengal Judicial Service. Suggested Test Series. FCI JE. Posted 7 months ago. Indian Coast Guard Assistant Commandant. AOC Material Assistant. Bihar ANM. Bihar Upper Primary Teacher. BEL Senior Engineer.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:.
West Bengal Judicial Service. Bihar Upper Primary Teacher. The 2s and the 2p would be filled then, we would have 2p6. Eugenics is the study of:. Puducherry Fireman. Indian Coast Guard Assistant Commandant. Now, why is six valence electrons interesting? RPSC 2nd Grade. RBI Grade B. Jharkhand High Court Assistant.

You commit an error. Let's discuss. Write to me in PM, we will communicate.
I think, that you are not right. I can defend the position.