How many valence electrons does n have
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry.
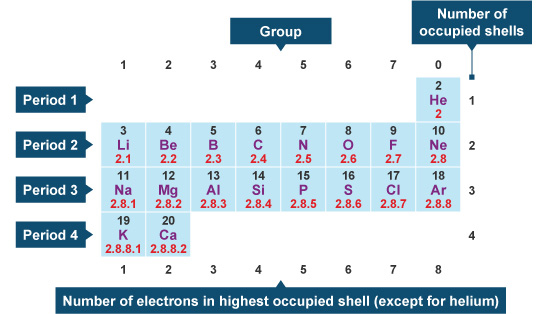
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals.
How many valence electrons does n have
Nitrogen has 5 valence electrons. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Each atom can thus complete its octet by sharing three electrons. Another thing to mention here is the fact that nitrogen's 5 valence electrons causes the atom to form 3- anions. This is the case because adding 3 electrons to nitrogen's valence shell will give it a complete octet. What is the number of valence electrons in nitrogen? Chemistry Electron Configuration Valence Electrons.
Organometallics on Ketones. Skip to main content.
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8,
In chemistry and physics , valence electrons are electrons in the outermost shell of an atom , and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond , a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element 's chemical properties, such as its valence —whether it may bond with other elements and, if so, how readily and with how many. In this way, a given element's reactivity is highly dependent upon its electronic configuration. For a main-group element , a valence electron can exist only in the outermost electron shell ; for a transition metal , a valence electron can also be in an inner shell.
How many valence electrons does n have
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Nitrogen is a non-metal element that occurs most abundantly in the atmosphere; nitrogen gas N 2 comprises
Snake coloring pages
Preparation of Organometallics. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. Drawing the Lewis Structure for N2H4. See all questions in Valence Electrons. Sharpless Epoxidation. Radical Reactions 1h 58m. You can reuse this answer Creative Commons License. Nitrides Nitrides are compounds of nitrogen with a less electronegative atom; in other words they are compounds with atoms that have a less full valence shell. Jun 8, Monosaccharides - Weak Oxidation Aldonic Acid. Before years ago, little was known about nitrogen. EAS:Nitration Mechanism.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
Energy Diagram. Alkyne Oxidative Cleavage. When mixed with water, nitride will form ammonia; the nitride ion acts as a very strong base. Molecular Representations 1h 14m. Naming Anhydrides. Almost all the oxides that form are gasses, and exist at 25 degrees Celsius. Good Leaving Groups. How can I calculate the valence electrons of transition metals? Double Elimination. Condensation Reactions. Naming Esters.

Excuse for that I interfere � At me a similar situation. It is possible to discuss. Write here or in PM.