How many electrons in f orbital
The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum:. For s, p, d, and f orbitals, how many electrons can each hold? Aug 11, See below.
The number denotes the energy level of the electron in the orbital. Thus 1 refers to the energy level closest to the nucleus; 2 refers to the next energy level further out, and so on. The letter refers to the shape of the orbital. The letters go in the order s, p, d, f, g, h, i, j, etc. The letters s, p, d, and f were assigned for historical reasons that need not concern us. All we have to do is remember the shapes that correspond to each letter.
How many electrons in f orbital
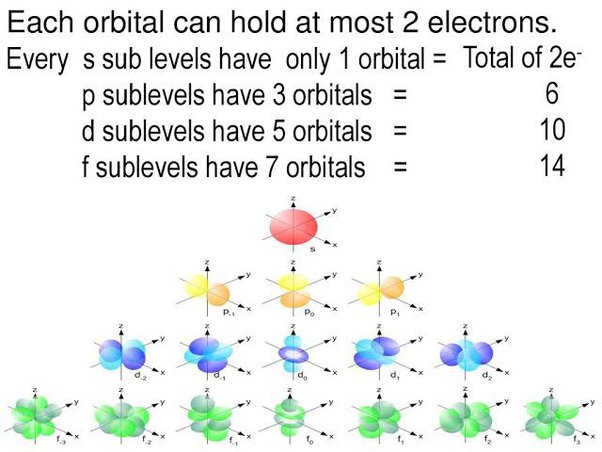
An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. There are multiple orbitals within an atom. Each has its own specific energy level and properties. Because each orbital is different, they are assigned specific quantum numbers : 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. This number indicates how many orbitals there are and thus how many electrons can reside in each atom. Orbitals that have the same or identical energy levels are referred to as degenerate. An example is the 2p orbital: 2p x has the same energy level as 2p y. This concept becomes more important when dealing with molecular orbitals. The Pauli exclusion principle states that no two electrons can have the same exact orbital configuration; in other words, the same quantum numbers. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.
There are 5 d orbitals in the d subshell.
.
The orbital letters are associated with the angular momentum quantum number, which is assigned an integer value from 0 to 3. The s correlates to 0, p to 1, d to 2, and f to 3. The angular momentum quantum number can be used to give the shapes of the electronic orbitals. The orbital names s , p , d , and f stand for names given to groups of lines originally noted in the spectra of the alkali metals. These line groups are called sharp , principal , diffuse , and fundamental.
How many electrons in f orbital
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons. The four different types of orbitals s,p,d, and f have different shapes, and one orbital can hold a maximum of two electrons.
Secret window stream
Question 7c3a9. What is the total number of f orbitals in an f subshell? What is the maximum number of electrons in an 3p subshell? Element X also has a partially filled 4d subshell. Since electrons all have the same charge, they stay as far away as possible because of repulsion. What type of orbitals do actinides and lanthanides mainly use? Thus, there are 3 angular nodes present. What are orbital probability patterns? Question What are some common mistakes students make with orbitals? The nucleus is the lobby where the protons and neutrons are, and in the floors above, we find the rooms orbitals with the electrons.
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties. The use of quantum theory provides the best understanding to these topics.
If there are more electrons after the 1s, and 2s orbitals have been filled, each p orbital will be filled with one electron first before two electrons try to reside in the same p orbital. There are two types of nodes, angular and radial nodes. Question 5ca Which sublevel is filled after the 5s sub level? Why do orbitals have different shapes? How many orbitals are in the 3d subshell? Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals. The second floor has the room styles s and p. What is the shape of the 3p atomic orbital? Question 8d7ed. Angular nodes are typically flat plane at fixed angles , like those in the diagram above. To sum up, the 3p z orbital has 2 nodes: 1 angular node and 1 radial node. A new Dictionary of Chemistry.

0 thoughts on “How many electrons in f orbital”