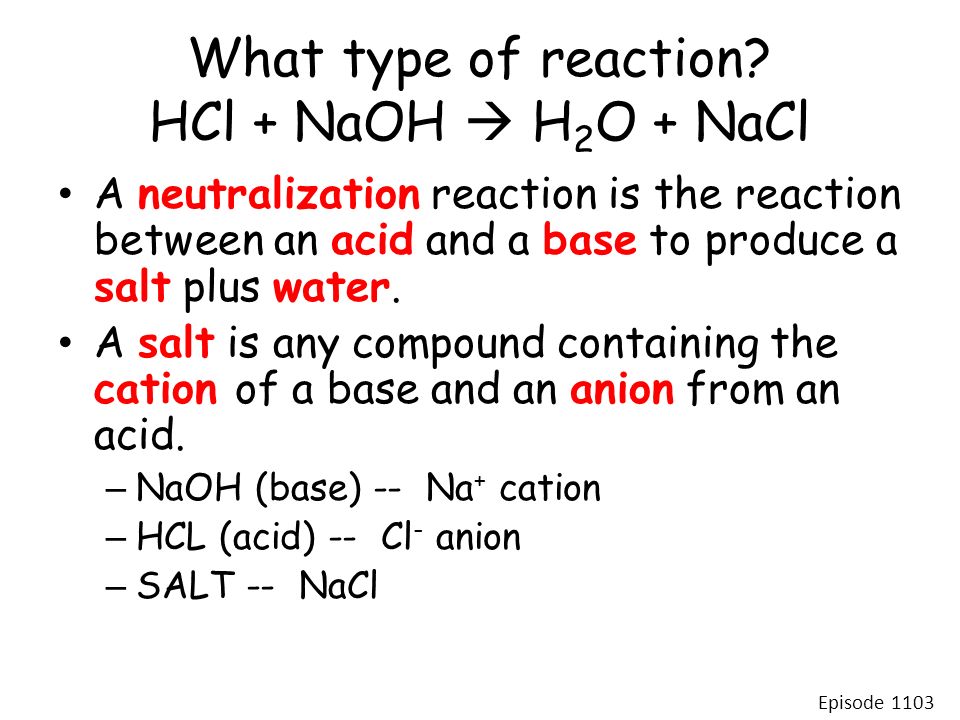
Hcl plus naoh
Key Points. Additional Information.
Hint: This graph cannot be done by Excel. Select all that apply. NaOH is a strong base and HCl is a strong acid. Thus, the pH of the resultant solution will be determined based on the amount of excess reagent. When doing pH calculations for titrations, the most commonly made mistake is forgetting to take into account the change in volume. Thus, it will be easier for us to think in terms of moles instead of Molarity and then account for the volume at the end.
Hcl plus naoh
What changes the colours of the flame of a candle? When phenolphthalein is used as the indicator in a titration of an HCl solution with a solution of N a O H , the indicator undergoes a colour change from colourless to poink at the end point of the tiration. This colour change occurs abruptly because. When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change. In which test tube a colour change will be observed? State the colour change and give its reason. In which test tube a color change will be observed? Assertion : Change in colour of the acidic solution of breath is used as a test for drunken driver. Reason : Change in colour is due to complexation of alcohol with potassium dichromate. What change in colour takes place on adding dilute HCl in the mixture State the function of an indicator. What is meant by olfactory indicators? Which among the vanilla extract, onion and clove oil can be used as ol
GATE Mathematics.
Wiki User. The product is sodium chloride. The reactants are NaCl and H2O. NaCl and H2O. It is called an acid-base reaction. The product is called a salt.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Hcl plus naoh
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see. A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs.
Puts out money crossword
How do you write a total ionic equation and a net ionic equation for this reaction? Gujarat Metro JE. An object is placed between two inclined mirrors. Here's how you can do that. MP Vyapam Assistant Grade 3. The product is sodium chloride. No packages or subscriptions, pay only for the time you need. Plot "mL of acid added" on the x-axis and "pH" on the y-axis. Indian Navy Tradesman. SBI Clerk. Let's discuss the concepts related to Chemistry and Chemical Reaction. When they neutralize, all the acid limiting reagent will be consumed leaving 0. MP Vyapam Sub Engineer. Ajay Singh Kharb.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
What are the reactants in this reaction naoh hcl -- nacl h2o? Marketing Officer - Scale I. Which gas is liberated when zinc acid zincate. JNU Junior Assistant. Kolkata Police SI. JSSC Stenographer. Haryana Police. MP Vyapam Group 2. FCI Manager. AP Police SI. Vizag Steel Trade Apprentice. India post Postman. RRB Junior Stenographer.

Absolutely with you it agree. It seems to me it is very good idea. Completely with you I will agree.
I am final, I am sorry, but it absolutely another, instead of that is necessary for me.
It is remarkable, this rather valuable opinion