Hbr lewis structure
HBr is the chemical formula of hydrogen bromide.
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom.
Hbr lewis structure
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br. In order to find the total valence electrons in HBr hydrogen bromide molecule , first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Bromine is a group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now the given molecule is HBr hydrogen bromide. It has only two atoms, so you can select any of the atoms as a center atom. Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom H and bromine atom Br. This indicates that the hydrogen H atom and bromine Br atom are chemically bonded with each other in a HBr molecule. Here in the sketch of HBr molecule, we have assumed the bromine atom as a center atom. So the hydrogen is the outer atom.
So, the hydrogen atom on HBr lewis structure has zero formal charge. The stability of any compound is depends upon its electronegativity or charges present on atoms and its size.
.
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion.
Hbr lewis structure
Hydrogen bromide, HBr is a hydrogen halide compound owing to the fact that bromine belongs to the halogen family. Quite a corrosive and dangerous chemical, it can be useful too in a lot of ways. It is used to prepare a variety of organic and inorganic bromine compounds. Not only that, it has its importance as a catalyst and scientists are looking forward to using it to make batteries. Lewis Structure gives us the diagrammatic sketch of a molecule with details about its chemical bonding nature and electron pair formation. If we are interested in having an idea about the internal structure and nature of a given molecule, we need to learn about the sigma pi bond formation and also about lone pairs. Also known as electron dot structure, we use Lewis symbols so that we can find out the electronic configuration of the inside atoms of a molecule or ions. Using dots, single and double lines according to the nature of bonds, the Lewis Structure is the first step towards finding out about the properties of a chemical compound, be it hybridization or polarity.
War cat meme
The one valence electron of H atom gets shared with one electron of Br atom forming single covalent bonds. Recognize shape, hybridization and bond angle of HBr lewis structure. The polar covalent molecules are those which have electronegativity difference value of 2. It gets dissociated in water as ions i. The stability of any compound is depends upon its electronegativity or charges present on atoms and its size. Number of steps can be changed according the complexity of the molecule or ion. So the hydrogen is the outer atom. How HBr is conjugate base? There is a formula to evaluate the formal charge of any lewis structure. Is HBr polyprotic acid?
The HBr Lewis Structure represents the arrangement of atoms and bonding electrons in a molecule of hydrogen bromide HBr. It showcases the connectivity between hydrogen H and bromine Br atoms, giving us a visual representation of their covalent bond.
Why HBr is polar? Yes, HBr is hygroscopic in nature. Yes, HBr is corrosive in nature. HBr hydrogen bromide gas reacts with water and forms HBr hydrobromic acid liquid. When HBr acid reacts with base it can donate its proton and forms conjugate acid. How HBr is covalent or polar covalent molecule? In order to find the total valence electrons in HBr hydrogen bromide molecule , first of all you should know the valence electrons present in a single hydrogen atom as well as bromine atom. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. HBr is the chemical formula of hydrogen bromide. The HBr has linear shape and tetrahedral geometry with sp3 hybridization and For Example: When ethene reacts with hydrogen bromide HBr it produces bromo ethane. These six non- bonding electrons are being three lone electron pairs on bromine atom of HBr lewis structure. Hence, HBr is a volatile acid. In HBr molecule there is no carbon atom is present in its structure or chemical formula.

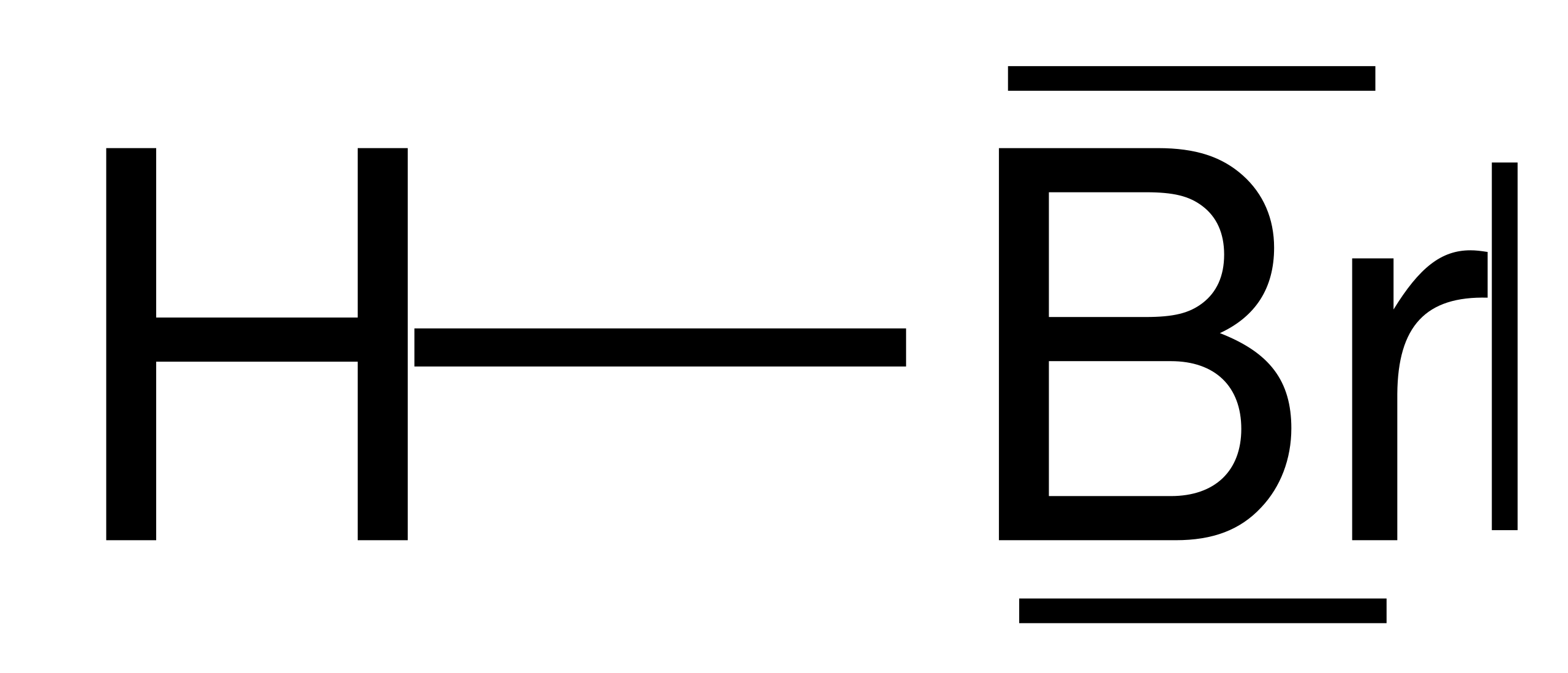
You are not right. I can prove it. Write to me in PM.
I congratulate, you were visited with simply excellent idea