H30+ lewis structure
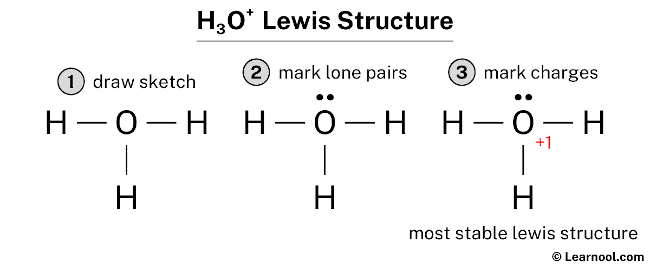
Hydronium ion contains hydrogen and oxygen atoms. Each hydrogen atom has linked with oxygen atom. Only one lone pair exist on oxygen atom. When we draw a lewis structure, there are several guidelines to follow.
In the periodic table , hydrogen lies in group 1, and oxygen lies in group Hence, hydrogen has one valence electron and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons and Oxygen valence electrons. We have a total of 8 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond.
H30+ lewis structure
There are 3 single bonds between the Oxygen atom O and each Hydrogen atom H. There is 1 lone pair on the Oxygen atom O. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. If we compare the electronegativity values of hydrogen H and oxygen O then the hydrogen atom is less electronegative.
Number of steps can be changed according the complexity of the molecule or ion. Remember that, there are total of four electron pairs.
.
Hydronium ion contains hydrogen and oxygen atoms. Each hydrogen atom has linked with oxygen atom. Only one lone pair exist on oxygen atom. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion.
H30+ lewis structure
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water. The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent. But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc. Knowing these basics will deepen our knowledge about this ion more. We should always try to know the background of any compound before studying any reaction regarding it. First of all, we need to calculate the total number of valence electrons present in hydronium ion.
Anal creampies eating
Total electron pairs are determined by dividing the number total valence electrons by two. So we have to only mark the remaining one electron pair as a lone pair on the sketch. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Your email address will not be published. You can see the number of bonding electrons and nonbonding electrons for each atom of H3O molecule in the image given below. Each hydrogen atom has linked with oxygen atom. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. There are two elements in hydronium ion; hydrogen and oxygen. But no need to mark on hydrogen, because each hydrogen has already two electrons. About author. Leave a Comment Cancel Reply Your email address will not be published.
The Oxygen atom O is at the center and it is surrounded by 3 Hydrogen atoms H.
Now in the H3O molecule, you have to put the electron pairs between the oxygen atom O and hydrogen atoms H. Save my name, email, and website in this browser for the next time I comment. Number of steps can be changed according the complexity of the molecule or ion. Hydronium ion contains hydrogen and oxygen atoms. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Hydrogen is group 1 element on the periodic table. And three O — H bonds are already marked. Read more about our Editorial process. Always start to mark the lone pairs from outside atoms. If we compare the electronegativity values of hydrogen H and oxygen O then the hydrogen atom is less electronegative. Here, the outside atoms are hydrogens.

In my opinion you are not right. I am assured. I suggest it to discuss. Write to me in PM, we will talk.
I congratulate, your idea is very good