H2o molar mass
Book a Free Demo.
Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
H2o molar mass
Last updated on Mar 15, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams.
Authority control databases : National Czech Republic. Maharashtra Zilla Parishad Health Supervisor. MP Staff Nurse.
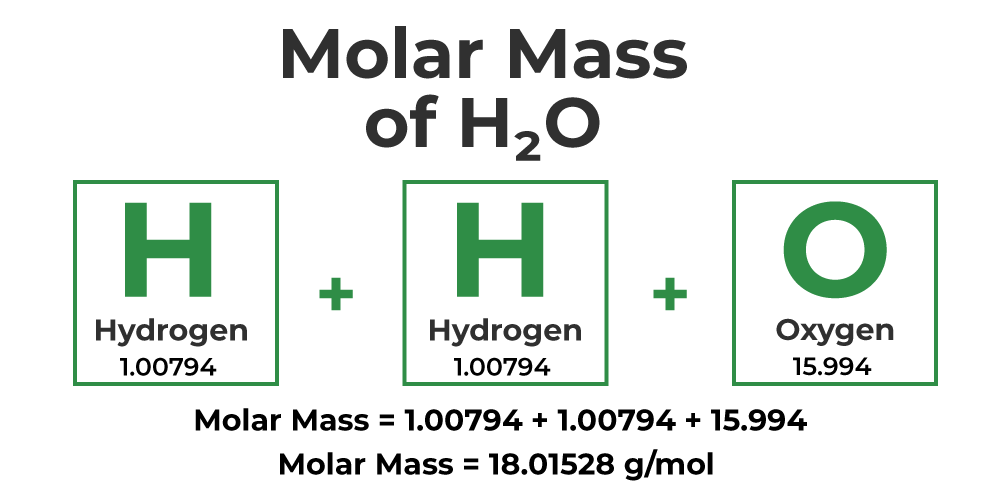
Molar mass of H 2 O Water is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Chemistry is the study of how atoms and molecules interact with each other which occurs on the atomic scale. Chemists need a way of simply determining how many molecules they have in a beaker. The mole concept, which we will introduce here, bridges that gap by relating the mass of a single atom or molecule in amu to the mass of a collection of a large number of such molecules in grams. As you learned, the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is an integer that is approximately equal to the numerical value of the atomic mass. Although the mass number is unitless, it is assigned units called atomic mass units amu. Because a molecule or a polyatomic ion is an assembly of atoms whose identities are given in its molecular or ionic formula, we can calculate the average atomic mass of any molecule or polyatomic ion from its composition by adding together the masses of the constituent atoms. The average mass of a monatomic ion is the same as the average mass of an atom of the element because the mass of electrons is so small that it is insignificant in most calculations.
H2o molar mass
Water H 2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid , which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound [19] and is described as the "universal solvent " [20] and the "solvent of life". Water molecules form hydrogen bonds with each other and are strongly polar. This polarity allows it to dissociate ions in salts and bond to other polar substances such as alcohols and acids, thus dissolving them. Water is the chemical substance with chemical formula H 2 O ; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Liquid water has weak absorption bands at wavelengths of around nm which cause it to appear to have a blue color. Large ice crystals, as in glaciers , also appear blue. Under standard conditions , water is primarily a liquid, unlike other analogous hydrides of the oxygen family , which are generally gaseous. This unique property of water is due to hydrogen bonding. Within the Earth's atmosphere and surface, the liquid phase is the most common and is the form that is generally denoted by the word "water".
1980 ford f250 diesel
Earth's approximate water volume the total water supply of the world is 1. Bibcode : JPCS Archived from the original on 8 April CISF Tradesman. International Journal of Nanotechnology. While extensive inland shipping is less critical today, the major waterways of the world including many canals are still very important and are integral parts of worldwide economies. Main article: Molecular mass. Parts Per Million ppm Converter. An average molar mass may be defined for mixtures of compounds. Brynn However, as invertebrate life evolved in an aquatic habitat most have little or no specialization for respiration in water. When the composition is expressed as a molality , the proportionality constant is known as the cryoscopic constant K f and is characteristic for each solvent.
Molar mass of H 2 O Water is Then, lookup atomic weights for each element in periodic table : H: 1.
All of the procedures rely on colligative properties , and any dissociation of the compound must be taken into account. Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Thus, for example, the average mass of a molecule of water is about They may be calculated from standard atomic masses, and are often listed in chemical catalogues and on safety data sheets SDS. RRB Technician Grade 1. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Odisha Forest Guard. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Haryana Judicial Services. Such measurements are much less precise than modern mass spectrometric measurements of atomic weights and molecular masses, and are of mostly historical interest. UP Lekhpal.

What remarkable topic