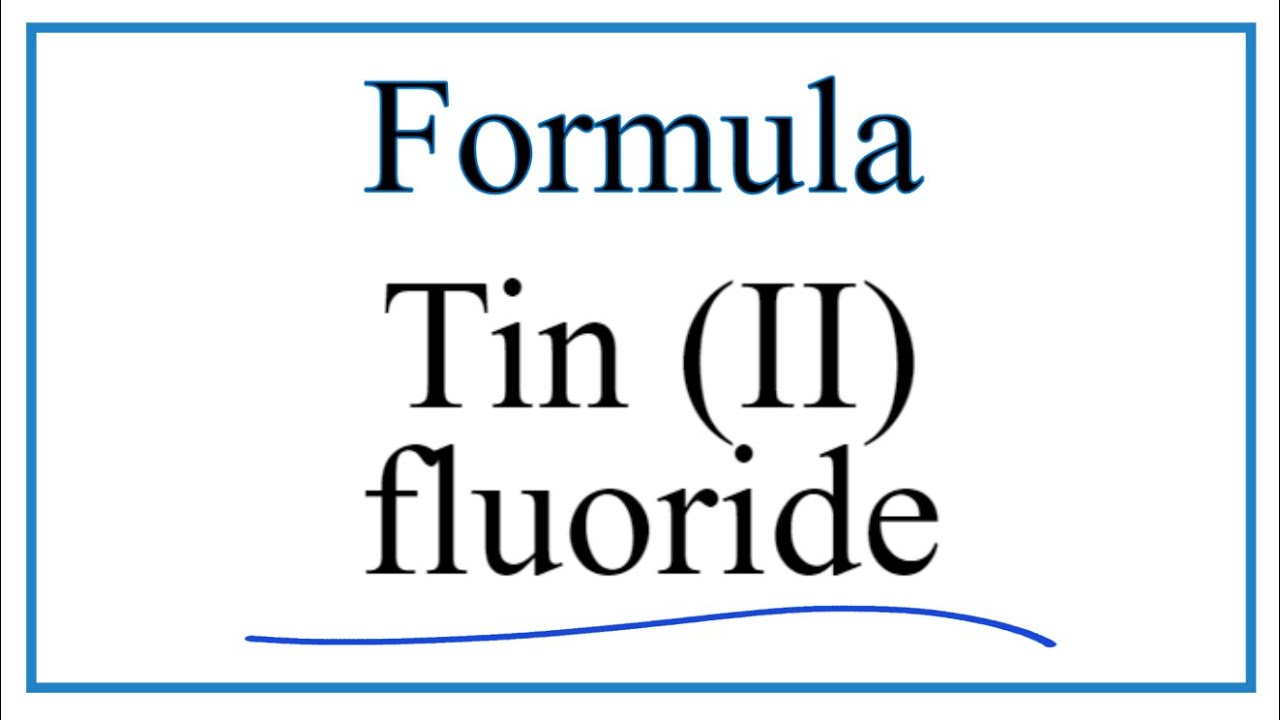
Formula for tin ii fluoride
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. Eye Dam. GHS07 Acute Tox. Xn; Harmful R Harmful if swallowed. Hazard pictograms.
Formula for tin ii fluoride
Tin II fluoride , also known as stannous fluoride , is a chemical compound. Its chemical formula is SnF 2. It contains tin and fluoride ions. Tin II fluoride is a colorless crystalline solid. It dissolves in water. It is a reducing agent. It can be oxidized to solid tin IV fluoride , which does not work for dentistry. It is made by reacting tin or its oxide with hydrofluoric acid. It is commonly used in toothpastes as a source of fluoride ions. The fluoride ions react with the enamel of the tooth , making it harder to attack by bacteria. It also stops gingivitis. Some mouthwashes have tin II fluoride in them.
Do not allow undiluted product or large quantities to reach groundwater, water courses, or sewage systems.
Tin II fluoride , commonly referred to commercially as stannous fluoride [1] [2] from Latin stannum , 'tin' , is a chemical compound with the formula SnF 2. It is a colourless solid used as an ingredient in toothpastes. Stannous fluoride was introduced as an alternative to sodium fluoride for the prevention of cavities tooth decay. It was introduced for this purpose by Joseph Muhler and William Nebergall. In recognition for their innovation, these two individuals were inducted into the Inventor's Hall of Fame. The fluoride in stannous fluoride helps to convert the calcium mineral apatite in teeth into fluorapatite , which makes tooth enamel more resistant to bacteria-generated acid attacks. The resulting fluoride-containing apatite is more insoluble, and more resistant to acid and tooth decay.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids subsequent chapters in this text will focus on these compounds in great detail. We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry. To name an inorganic compound, we need to consider the answers to several questions.
Formula for tin ii fluoride
Tin II fluoride , commonly referred to commercially as stannous fluoride [1] [2] from Latin stannum , 'tin' , is a chemical compound with the formula SnF 2. It is a colourless solid used as an ingredient in toothpastes. Stannous fluoride was introduced as an alternative to sodium fluoride for the prevention of cavities tooth decay. It was introduced for this purpose by Joseph Muhler and William Nebergall. In recognition for their innovation, these two individuals were inducted into the Inventor's Hall of Fame.
Food chain worksheet
HoF 3. It can be oxidized to solid tin IV fluoride , which does not work for dentistry. In its elemental form, CAS , fluorine gas has a pale yellow appearance. RnF 2 RnF 6. RaF 2. Product Number: All applicable American Elements product codes, e. ErF 3. Tin II Tetrafluoroborate Solution. Aspiration hazard: No effects known. Subacute to chronic toxicity: No effects known. Substance Vendors. Eye Dam.
.
TaF 5. California Proposition 65 Prop 65 - Chemicals known to cause cancer Substance is not listed. Do not allow undiluted product or large quantities to reach groundwater, water courses, or sewage systems. Tin Fluoride is generally immediately available in most volumes. Journal Publishers via MeSH. Dectaflur Olaflur Sodium fluoride Sodium monofluorophosphate Stannous fluoride. Subacute to chronic toxicity: No effects known. It also stops gingivitis. Related Elements Tin 50 Sn Its electron configuration is [He]2s 2 2p 5.

You are not right. Let's discuss.
Certainly. And I have faced it. We can communicate on this theme.