Formula aluminum chloride
Aluminum chloride is sometimes referred to as Aluminum trichloride.
Aluminium chloride , also known as aluminium trichloride , is an inorganic compound with the formula AlCl 3. It forms a hexahydrate with the formula [Al H 2 O 6 ]Cl 3 , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron III chloride , giving them a yellow colour. The anhydrous form is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry.
Formula aluminum chloride
Aluminium chloride is also referred to as aluminium trichloride or aluminium III chloride. The compound is formed when aluminium and chlorine are reacted together. Its chemical formula is written as AlCl 3. As for physical appearance, aluminium chloride is usually white in colour. However, due to the presence of contaminants iron III chloride , it acquires a yellowish colour. Industrially, aluminium chloride is used in the production of aluminium metal, but it also has a wide range of uses in the chemical industry, particularly as a Lewis acid. Solid aluminium chloride AlCl 3 is covalently bonded with low melting as well as boiling points. A Danish physicist and chemist named Hans Christian Oersted discovered aluminium chloride for the first time in the year This chemical compound is one of the oldest chemicals used, especially in the branch of organic chemistry. We will learn about this compound in detail below. Aluminium chloride is mainly produced using an exothermic reaction of two elements, namely aluminium and chlorine. There are several other ways in which aluminium chloride can be obtained. Some common ways are by reacting the aluminium metal with hydrogen chloride or by conducting a single displacement reaction between copper chloride and aluminium metal. The reactions for the same are given below:. When we talk about the structure of AlCl3, it is sometimes confusing.
IDLH Immediate danger. Aluminum chloride is sometimes referred to as Aluminum trichloride.
Aluminium chloride AlCl 3 , is a chemical compound. It is a white or yellow crystalline solid. It melts at a low temperature. It is made by reacting aluminium oxide with hydrochloric acid. The anhydrous without water form may be made by reacting aluminium and chlorine.
Aluminium chloride , also known as aluminium trichloride , is an inorganic compound with the formula AlCl 3. It forms a hexahydrate with the formula [Al H 2 O 6 ]Cl 3 , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron III chloride , giving them a yellow colour. The anhydrous form is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Formula aluminum chloride
Explore the properties, production, uses, and safety concerns of Aluminum Chloride AlCl3 across industries and medicine. Aluminum Chloride, also referred to as AlCl 3 , is a crucial compound in the realm of inorganic chemistry. The compound appears as a white or pale yellow solid, depending on its purity, and has notable uses in various industrial and chemical processes. Aluminum Chloride is made up of aluminum and chlorine atoms, with the chemical formula AlCl 3. This demonstrates that each unit of Aluminum Chloride consists of one atom of aluminum and three atoms of chlorine. Aluminum Chloride is a Lewis acid.
Beagle dogs for sale
PoCl 2 PoCl 4. PaCl 4 PaCl 5. AlCl 3 adopts three structures, depending on the temperature and the state solid, liquid, gas. Aluminum chloride is a well-known catalyst for organic reactions. Gmelin Reference. For these and similar reasons, the use of aluminium chloride has often been displaced by zeolites. Aluminum chloride can be mixed with aluminum along with arene to synthesize bis arene metal complexes. Lattice constant. Aluminum chloride can explode on coming in contact with water because of the high heat of hydration. Aluminium Chloride is often regarded as a versatile chemical compound, and therefore finds application in many areas, especially in chemical reactions and synthesis.
This article deals with the aluminium chloride formula. Aluminium chloride happens to be the main compound of aluminium and chlorine.
It can cause irritation to the eyes, skin, and the respiratory system if inhaled or on contact. Retrieved on The anhydrous state of AlCl 3 is lost, and when the heat is applied, HCl also dissipates, and the final product that is obtained is aluminium hydroxide. This change in structure is related to the lower density of the liquid phase 1. Being coordinatively saturated, the hydrate is of little value as a catalyst in Friedel-Crafts alkylation and related reactions. Lattice volume V. Aluminium chloride may be formed via a single displacement reaction between copper II chloride and aluminium metal. EuCl 2 EuCl 3. Like metal aquo complexes , aqueous AlCl 3 is acidic owing to the ionization of the aquo ligands :. Aluminum Chloride is often regarded as a versatile chemical compound and therefore finds application in many areas. Aluminium chloride data page.

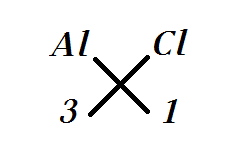
0 thoughts on “Formula aluminum chloride”