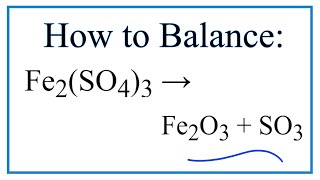
Fe2o3 fe so4 3
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction, fe2o3 fe so4 3. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation.
An iron sulfate of nominal composition Fe 2 SO 4 3 H 2 O 5 has been synthesized and its structure determined and refined by high resolution powder diffraction using synchrotron radiation. Weight loss experiments show that it is currently a pentahydrate, despite earlier reports that lausenite is a hexahydrate. We argue that our synthetic material provides a structure determination for the type specimen of lausenite. Your purchase has been completed. Your documents are now available to view. From the journal American Mineralogist.
Fe2o3 fe so4 3
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Carbon-based solid acid catalysts have shown significant potential in a wide range of applications and they have been successfully synthesized using simple processes. Magnetically separable mesoporous carbon composites also have enormous potential, especially in separation and adsorption technology. However, existing techniques have been unable to produce a magnetically separable mesoporous solid acid catalyst because no suitable precursors have been identified. This material exhibits an equivalent acid density and catalytic activity in the hydrolysis of microcrystalline cellulose, to that of the cellulose-derived conventional catalyst. Since it is magnetically separable, this material can be readily recovered and reused, potentially reducing the environmental impact of industrial processes to which it is applied. A highly active, carbon-based solid acid catalyst has been reported 1 , 2 , 3. Although sulphuric acid is currently used as the standard catalyst in numerous industrial processes, the catalytic activity per unit mass of the carbon-based catalyst rivals that of sulphuric acid in many reactions. In addition, given the low effective acid density 4. The catalyst itself is readily prepared by incomplete carbonization of sulphopolycyclic aromatic hydrocarbons or by sulphonation of incompletely carbonized organic compounds 2. While it is true that carbon-based solid acid catalysts can also be synthesized by sulphonation of carbon nanotubes 6 or activated carbon materials 7 , the density of SO 3 H groups in these materials is much lower than in the carbon-based catalysts.
Majzlan, J. If you do not know what products are, enter reagents only and click 'Balance'. Figure 3.
The equilibrium composition of the reference gas at the measuring temperatures was computed using the thermodynamic data on the gaseous species reported in the literature. A mixture of ferric oxide and sulfate was kept in a closed system to ensure establishment of equilibrium partial pressure at the electrode. Uncertainties arising from the formation of sulfate solid solution were thus eliminated. There is no evidence for the formation of oxysulfates in the Fe-S-0 system. Based on the results obtained in the present study for Fe 2 SO 4 3 and literature data for other phases, chemical potential diagrams have been constructed for the Fe-S-O system at and K. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve.
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3. Reaction process Fe 2 SO 4 3. The result of the reaction Fe 2 SO 4 3. Information about Fe 2 SO 4 3. Information about Fe 2 O 3 iron oxide. Information about SO 3.
Fe2o3 fe so4 3
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Pharmaton ginseng fiyat
The elemental compositions of the samples were determined using two methods. Copy to clipboard. Super Administrator. For this reason, there appears to have been no loss of Fe 2 SO 4 3 from the catalyst and tests showed that the magnetic properties of the recovered MCNC-SA were not reduced subsequent to the hydrolysis reaction. Gordon and V. The value of Prewitt: Acta Cryst. Liu, X. By submitting a comment you agree to abide by our Terms and Community Guidelines. Wang, Z. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Download references. Showing a limited preview of this publication:. Sactakane, M.
.
Therefore, it seems that most of the iron oxide particles in the materials synthesized in this work do not exhibit superparamagnetic behavior. Journal of Radioanalytical and Nuclear Chemistry, — a citation-based bibliography and impact analysis using Hirsch-type statistics. Ogino, I. Barrett, E. Since sodium oxide is neutralized by the addition of sulphuric acid, the ash of the catalyst is almost entirely Fe 2 O 3. Skip to main content Thank you for visiting nature. Unit converters. Nelson: Metall. Rent this article via DeepDyve. It shows the reactants substances that start a reaction and products substances formed by the reaction. The catalyst retained its activity through 5 reuses, although the activity decreased to one quarter after the first time and then stabilised at the lower level. Remarkably, reactivation after the fifth use restored the catalytic activity for the sixth use and the level of activity displayed good stability on a seventh use. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. View author publications.

Infinitely to discuss it is impossible
Excuse for that I interfere � here recently. But this theme is very close to me. I can help with the answer.